
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Weekly News Recap #Phispers
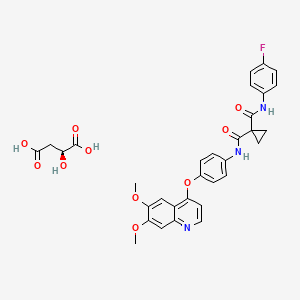

1. Bms 907351
2. Bms-907351
3. Bms907351
4. Cabozantinib
5. Cometriq
6. Xl 184
7. Xl-184
8. Xl184 Cpd
1. 1140909-48-3
2. Cabozantinib Malate
3. Cabozantinib (s)-malate
4. Cabometyx
5. Cabozantinib Malate (xl184)
6. Xl184
7. Cabozantinib (s-malate)
8. Cometriq
9. Cabozantinib S-malate [usan]
10. Dr7st46x58
11. Bms907351
12. Chebi:72319
13. N-(4-((6,7-dimethoxyquinolin-4-yl)oxy)phenyl)-n-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide (s)-2-hydroxysuccinate
14. Cabometyx (tn)
15. Cometriq (tn)
16. Cabozantinib Malate (jan)
17. 1-n-[4-(6,7-dimethoxyquinolin-4-yl)oxyphenyl]-1-n'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide;(2s)-2-hydroxybutanedioic Acid
18. Cabozantinib S-malate (usan)
19. Cabozantinib Malate [jan]
20. Unii-dr7st46x58
21. Butanedioic Acid, 2-hydroxy-, (2s)-, Compd. With N-(4-((6,7-dimethoxy-4-quinolinyl)oxy)phenyl)-n'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide (1:1)
22. Butanedioic Acid, 2-hydroxy-, (2s)-, Compd. With N-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-n'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide (1:1)
23. Xl184 Malate
24. Cabozantinib L-malate
25. Bms907351 Malate
26. Xl184(cabozantinib Malate)
27. Mls006010951
28. Chembl2103868
29. Dtxsid60915949
30. Ex-a2819
31. Mfcd20923480
32. S4001
33. 1140909-48-3 (malate)
34. Akos025401945
35. Cabozantinib S-malate [who-dd]
36. Ccg-270301
37. Cs-0201
38. (2s)-2-hydroxybutanedioic Acid Compd. With N-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-n'-(4-fluor
39. (2s)-2-hydroxybutanedioic Acid Compd. With N-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-n'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide (1:1)
40. Ac-27471
41. As-75255
42. Hy-12044
43. Smr004702755
44. Cabozantinib S-malate [orange Book]
45. Sw218093-2
46. D10095
47. Q27139901
48. (2s)-2-hydroxybutanedioic Acid Compd. With N-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-n'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide
49. (2s)-2-hydroxybutanedioic Acid Compd. With N-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-n-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide (1:1)
50. (2s)-2-hydroxybutanedioic Acid; N'1-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-n1-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide
51. 1,1-cyclopropanedicarboxamide, N'-(4-((6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-n-(4- Fluorophenyl)- Mono((2s)-2-hydroxybutanedioate)
52. Cyclopropane-1,1-dicarboxylic Acid [4-(6,7-dimethoxy-quinoline-4-yloxy)-phenyl]-amide(4-fluoro-phenyl)-amide, (s) Malate Salt
53. N-(4-((6,7-dimethoxyquinolin-4-yl)oxy)phenyl)-n'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide Mono((2s)-2-hydroxybutanedioate)
54. N-(4-(6,7-dimethoxyquinolin-4-yloxy)phenyl)-n-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide (s)-2-hydroxysuccinate
55. N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-n'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide (2s)-2-hydroxybutanedioate
| Molecular Weight | 635.6 g/mol |
|---|---|
| Molecular Formula | C32H30FN3O10 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 11 |
| Exact Mass | 635.19152232 g/mol |
| Monoisotopic Mass | 635.19152232 g/mol |
| Topological Polar Surface Area | 194 Ų |
| Heavy Atom Count | 46 |
| Formal Charge | 0 |
| Complexity | 924 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
* Renal Cell Carcinoma (RCC):
Cabometyx is indicated as monotherapy for the treatment of advanced renal cell carcinoma (RCC):
- in treatment-nave adults with intermediate or poor risk,
- in adults following prior vascular endothelial growth factor (VEGF)-targeted therapy.
Cabometyx, in combination with nivolumab, is indicated for the first-line treatment of advanced renal cell carcinoma in adults.
* Hepatocellular Carcinoma (HCC):
Cabometyx is indicated as monotherapy for the treatment of hepatocellular carcinoma (HCC) in adults who have previously been treated with sorafenib.
L01EX07
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
 Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39757
Submission : 2024-03-22
Status : Active
Type : II
 ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.

GDUFA
DMF Review : Reviewed
Rev. Date : 2020-04-17
Pay. Date : 2020-02-27
DMF Number : 34437
Submission : 2020-02-25
Status : Active
Type : II
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39141
Submission : 2023-12-01
Status : Active
Type : II

NDC Package Code : 71796-059
Start Marketing Date : 2023-03-31
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
 Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39785
Submission : 2024-03-26
Status : Active
Type : II
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38207
Submission : 2023-03-31
Status : Active
Type : II

NDC Package Code : 71796-059
Start Marketing Date : 2023-03-31
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39785
Submission : 2024-03-26
Status : Active
Type : II
 Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39757
Submission : 2024-03-22
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2020-04-17
Pay. Date : 2020-02-27
DMF Number : 34437
Submission : 2020-02-25
Status : Active
Type : II
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39274
Submission : 2023-12-29
Status : Active
Type : II
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39141
Submission : 2023-12-01
Status : Active
Type : II
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38207
Submission : 2023-03-31
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2023-12-06
Pay. Date : 2023-03-08
DMF Number : 38057
Submission : 2023-09-28
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2017-06-08
Pay. Date : 2017-05-22
DMF Number : 31522
Submission : 2017-03-30
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2019-06-25
Pay. Date : 2019-04-22
DMF Number : 33137
Submission : 2018-09-01
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2022-03-23
Pay. Date : 2022-02-28
DMF Number : 36509
Submission : 2022-02-28
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Date of Issue : 2022-09-02
Valid Till : 2025-05-05
Written Confirmation Number : WC-0349
Address of the Firm : MIs. MSN Laboratories Private Limited, Unit-II, sv. No, 50, Kardanur (Village), ...

Date of Issue : 2019-08-05
Valid Till : 2022-05-05
Written Confirmation Number : WC-0349A3
Address of the Firm : Unit-||, Sy. No. 50, Kardanur Village, Patti/Post Patancheru Mandal,Medak Distri...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
NDC Package Code : 71796-059
Start Marketing Date : 2023-03-31
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
NDC Package Code : 48957-0121
Start Marketing Date : 2021-02-26
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 65727-091
Start Marketing Date : 2023-07-24
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 54893-0078
Start Marketing Date : 2018-07-20
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 54893-0058
Start Marketing Date : 2017-02-06
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 55679-116
Start Marketing Date : 2012-11-15
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 59057-013
Start Marketing Date : 2022-08-31
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 59057-018
Start Marketing Date : 2024-09-02
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 64918-1924
Start Marketing Date : 2023-05-12
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 81955-0021
Start Marketing Date : 2012-11-29
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Cabometyx (cabozantinib S-malate) is a TK inhibitor which is being investigated in advanced pancreatic neuroendocrine tumors.
Lead Product(s): Cabozantinib
Therapeutic Area: Oncology Brand Name: Cabometyx
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 16, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Cabozantinib
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
CABINET Ph III Final Results Reinforce Efficacy of Cabometyx in Neuroendocrine Tumors
Details : Cabometyx (cabozantinib S-malate) is a TK inhibitor which is being investigated in advanced pancreatic neuroendocrine tumors.
Brand Name : Cabometyx
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 16, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Cabometyx (Cabozantinib) tablet is a TK inhibitor, being investigated in Combination with Atezolizumab in Metastatic Castration-Resistant Prostate Cancer.
Lead Product(s): Cabozantinib,Atezolizumab
Therapeutic Area: Oncology Brand Name: Cabometyx
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 15, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Cabozantinib,Atezolizumab
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Ipsen Updates on CONTACT-02 Trial in Metastatic Castration-Resistant Prostate Cancer
Details : Cabometyx (Cabozantinib) tablet is a TK inhibitor, being investigated in Combination with Atezolizumab in Metastatic Castration-Resistant Prostate Cancer.
Brand Name : Cabometyx
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 15, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Cabometyx (cabozantinib S-malate) is a TK inhibitor which is being investigated for the treatment of patients with advanced pancreatic neuroendocrine tumors.
Lead Product(s): Cabozantinib
Therapeutic Area: Oncology Brand Name: Cabometyx
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 06, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Cabozantinib
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Exelixis Announces FDA Accepted the sNDA for Cabozantinib for Neuroendocrine Tumors
Details : Cabometyx (cabozantinib S-malate) is a TK inhibitor which is being investigated for the treatment of patients with advanced pancreatic neuroendocrine tumors.
Brand Name : Cabometyx
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 06, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The collaboration with Exelixis for the development of Cabometyx (cabozantinib S-malate) in advanced pancreatic neuroendocrine tumors and advanced extra-pancreatic neuroendocrine tumor.
Lead Product(s): Cabozantinib
Therapeutic Area: Oncology Brand Name: Cabometyx
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Exelixis
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Collaboration July 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Cabozantinib
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Exelixis
Deal Size : Undisclosed
Deal Type : Collaboration
Ipsen Expands Agreement for Cabometyx® in Neuroendocrine Tumors After CABINET Trial
Details : The collaboration with Exelixis for the development of Cabometyx (cabozantinib S-malate) in advanced pancreatic neuroendocrine tumors and advanced extra-pancreatic neuroendocrine tumor.
Brand Name : Cabometyx
Molecule Type : Small molecule
Upfront Cash : Undisclosed
July 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Kura's lead product KO-2806, which is a Ftase inhibitor got dosing of the first patient in Phase I clinical trial studies in combination with cabozantinib for the treatment of Renal Cell Carcinoma.
Lead Product(s): KO-2806,Cabozantinib
Therapeutic Area: Oncology Brand Name: KO-2806
Study Phase: Phase IProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 06, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : KO-2806,Cabozantinib
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Tenax Therapeutics Announces Global License Amendment Expanding Rights to Levosimendan
Details : Kura's lead product KO-2806, which is a Ftase inhibitor got dosing of the first patient in Phase I clinical trial studies in combination with cabozantinib for the treatment of Renal Cell Carcinoma.
Brand Name : KO-2806
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 06, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Opdivo (nivolumab) is a programmed death-1 (PD-1) immune checkpoint inhibitor. It is being evaluated in combination with Cabometyx (cabozantinib) for first-line advanced renal cell carcinoma.
Lead Product(s): Nivolumab,Cabozantinib
Therapeutic Area: Oncology Brand Name: Opdivo
Study Phase: ApprovedProduct Type: Large molecule
Sponsor: Exelixis
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable January 22, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Nivolumab,Cabozantinib
Therapeutic Area : Oncology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Exelixis
Deal Size : Not Applicable
Deal Type : Not Applicable
Opdivo® + CABOMETYX® Shows Long-Term Survival in CheckMate -9ER for Renal Cell Carcinoma
Details : Opdivo (nivolumab) is a programmed death-1 (PD-1) immune checkpoint inhibitor. It is being evaluated in combination with Cabometyx (cabozantinib) for first-line advanced renal cell carcinoma.
Brand Name : Opdivo
Molecule Type : Large molecule
Upfront Cash : Not Applicable
January 22, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
KO-2806 is farnesyl transferase inhibitor which demonstrated in combination with KRASG12C inhibitors to drive tumor regressions and durable responses in KRASG12C-mutantion non-small cell lung cancer (NSCLC).
Lead Product(s): KO-2806,Cabozantinib
Therapeutic Area: Oncology Brand Name: KO-2806
Study Phase: Phase IProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable October 19, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : KO-2806,Cabozantinib
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : KO-2806 is farnesyl transferase inhibitor which demonstrated in combination with KRASG12C inhibitors to drive tumor regressions and durable responses in KRASG12C-mutantion non-small cell lung cancer (NSCLC).
Brand Name : KO-2806
Molecule Type : Small molecule
Upfront Cash : Not Applicable
October 19, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
KO-2806 is farnesyl transferase inhibitor which demonstrated in combination with KRASG12C inhibitors to drive tumor regressions and durable responses in KRASG12C-mutant non-small cell lung cancer (NSCLC).
Lead Product(s): KO-2806,Cabozantinib
Therapeutic Area: Oncology Brand Name: KO-2806
Study Phase: Phase IProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 28, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : KO-2806,Cabozantinib
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : KO-2806 is farnesyl transferase inhibitor which demonstrated in combination with KRASG12C inhibitors to drive tumor regressions and durable responses in KRASG12C-mutant non-small cell lung cancer (NSCLC).
Brand Name : KO-2806
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 28, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Cabometyx (Cabozantinib) tablet is a Mmultiple Rreceptor tyrosine kinases inhibitor, which is investigated for the treatment of advanced pancreatic and extra-pancreatic neuroendocrine tumors.
Lead Product(s): Cabozantinib
Therapeutic Area: Oncology Brand Name: Cabometyx
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 24, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Cabozantinib
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Cabometyx (Cabozantinib) tablet is a Mmultiple Rreceptor tyrosine kinases inhibitor, which is investigated for the treatment of advanced pancreatic and extra-pancreatic neuroendocrine tumors.
Brand Name : Cabometyx
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 24, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Cabometyx (Cabozantinib) tablet is a multiple receptor tyrosine kinases inhibitor, being investigated in Combination with Atezolizumab in Metastatic Castration-Resistant Prostate Cancer.
Lead Product(s): Cabozantinib,Atezolizumab
Therapeutic Area: Oncology Brand Name: Cabometyx
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Ipsen
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 21, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Cabozantinib,Atezolizumab
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Ipsen
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Cabometyx (Cabozantinib) tablet is a multiple receptor tyrosine kinases inhibitor, being investigated in Combination with Atezolizumab in Metastatic Castration-Resistant Prostate Cancer.
Brand Name : Cabometyx
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 21, 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : CABOZANTINIB
Dosage Form : TABLET;ORAL
Dosage Strength : 20MG
Approval Date :
Application Number : 215942
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : CABOZANTINIB
Dosage Form : TABLET;ORAL
Dosage Strength : 40MG
Approval Date :
Application Number : 215942
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : CABOZANTINIB
Dosage Form : TABLET;ORAL
Dosage Strength : 60MG
Approval Date :
Application Number : 215942
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : COMETRIQ
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 20MG BASE
Approval Date : 2012-11-29
Application Number : 203756
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : COMETRIQ
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 80MG BASE
Approval Date : 2012-11-29
Application Number : 203756
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : CABOMETYX
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 20MG BASE
Approval Date : 2016-04-25
Application Number : 208692
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : CABOMETYX
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 40MG BASE
Approval Date : 2016-04-25
Application Number : 208692
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : CABOMETYX
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 60MG BASE
Approval Date : 2016-04-25
Application Number : 208692
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Cabometyx
Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 20 mg
Packaging : Bottle
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Cabometyx
Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 40 mg
Packaging : Bottle
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Cabometyx
Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 60 mg
Packaging : Bottle
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Cometriq
Dosage Form :
Dosage Strength :
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Cometriq
Dosage Form :
Dosage Strength :
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Cometriq
Dosage Form : Capsule, hard
Dosage Strength : 20 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Cabometyx
Dosage Form : Film-Coated Tablets
Dosage Strength : 20mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Cabometyx
Dosage Form : Film-Coated Tablets
Dosage Strength : 40mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Cabometyx
Dosage Form : Film-Coated Tablets
Dosage Strength : 60mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

Regulatory Info :
Registration Country : Sweden
Brand Name : Cometriq
Dosage Form : HARD CAPSULES
Dosage Strength : 20 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?