
Synopsis
0
EU WC
0
VMF
0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
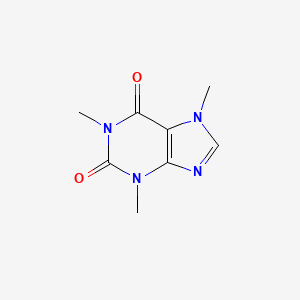

1. 1,3,7-trimethylxanthine
2. Caffedrine
3. Coffeinum N
4. Coffeinum Purrum
5. Dexitac
6. Durvitan
7. No Doz
8. Percoffedrinol N
9. Percutafine
10. Quick Pep
11. Quick-pep
12. Quickpep
13. Vivarin
1. 58-08-2
2. Guaranine
3. 1,3,7-trimethylxanthine
4. Methyltheobromine
5. Theine
6. Thein
7. Cafeina
8. Koffein
9. Mateina
10. Alert-pep
11. Caffein
12. Cafipel
13. Coffeine
14. Refresh'n
15. Caffedrine
16. Vivarin
17. Anhydrous Caffeine
18. Stim
19. Cafamil
20. Cafecon
21. Caffine
22. Dexitac
23. Nodaca
24. No-doz
25. Eldiatric C
26. 7-methyltheophylline
27. Durvitan
28. Hycomine
29. Organex
30. Nix Nap
31. Methyltheobromide
32. Coffeinum
33. Coffein
34. Phensal
35. 1,3,7-trimethylpurine-2,6-dione
36. 3,7-dihydro-1,3,7-trimethyl-1h-purine-2,6-dione
37. Caffeine, Synthetic
38. Quick-pep
39. Synalgos
40. Tirend
41. 1,3,7-trimethyl-2,6-dioxopurine
42. Theophylline, 7-methyl
43. Dhcplus
44. Tri-aqua
45. 1h-purine-2,6-dione, 3,7-dihydro-1,3,7-trimethyl-
46. Kofein
47. Miudol
48. Caffeine, Anhydrous
49. Theobromine, 1-methyl-
50. Propoxyphene Compound 65
51. 1,3,7-trimethyl-3,7-dihydro-1h-purine-2,6-dione
52. Kofein [czech]
53. Coffein [german]
54. Koffein [german]
55. 1-methyltheobromine
56. Caffeine (natural)
57. Xanthine, 1,3,7-trimethyl
58. Caffeina [italian]
59. Theophylline Me
60. Methylxanthine Theophylline
61. Theobromine Me
62. Nci-c02733
63. Sk-65 Compound
64. Anacin
65. Anacin Maximum Strength
66. Caffeine Anhydrous
67. Fema No. 2224
68. P-a-c Analgesic Tablets
69. C8h10n4o2
70. Hsdb 36
71. Caffeinum
72. Brn 0017705
73. 1,3,7-trimethyl-2,3,6,7-tetrahydro-1h-purine-2,6-dione
74. Nsc 5036
75. A.s.a. And Codeine Compound
76. 1,3,7-trimethyl-1h-purine-2,6(3h,7h)-dione
77. Ai3-20154
78. Nsc-5036
79. Chembl113
80. Caffeine Melting Point Standard
81. Cff
82. Chebi:27732
83. 3g6a5w338e
84. Mfcd00005758
85. Caffenium
86. Sk 65 Compound
87. Caffeina
88. Dsstox_cid_232
89. Caffeine [ban:jan]
90. Caffeine-d3
91. Dsstox_rid_75448
92. Dsstox_gsid_20232
93. Cafeine
94. Teina
95. Cas-58-08-2
96. Caffeine (usp)
97. Smr000326667
98. Ccris 1314
99. Sr-01000075187
100. Anhydrous Caffeine (tn)
101. Einecs 200-362-1
102. Monomethyl Derivative Of Theophylline
103. Caffeine [usp:ban:jan]
104. Anhydrous Caffeine (jp15)
105. Unii-3g6a5w338e
106. Caffeine Hydrous
107. 1gfz
108. Caffeine, Bioxtra
109. Tnp00310
110. Monohydrate Caffeine
111. Respia (tn)
112. 1-methyl-theobromine
113. 7-methyl Theophylline
114. Cafergot (salt/mix)
115. 1,7-trimethylxanthine
116. Spectrum_001301
117. 1l5q
118. 1l7x
119. 2a3b
120. 3g6m
121. 3,7-dihydro-1,3,7-trimethyl-1h-purine
122. Caffeine [fhfi]
123. Caffeine [hsdb]
124. Caffeine [iarc]
125. Caffeine [inci]
126. Caffeine [fcc]
127. Xanthine,3,7-trimethyl
128. Caffeine [ii]
129. Caffeine [mi]
130. Theine, Methyltheobromine
131. Caffeine [vandf]
132. Caffeinum [hpus]
133. 1,3,7-trimethylxantine
134. Spectrum2_001261
135. Spectrum3_000321
136. Spectrum4_001782
137. Spectrum5_000423
138. Lopac-c-0750
139. Caffeine [mart.]
140. Bmse000206
141. Caffeine [usp-rs]
142. Caffeine [who-dd]
143. Caffeine [who-ip]
144. Molmap_000054
145. Probes1_000150
146. Probes2_000128
147. C 0750
148. Ec 200-362-1
149. Schembl5671
150. Anhydrous Caffeine (jp17)
151. Nciopen2_008255
152. Bidd:pxr0172
153. Lopac0_000228
154. 1, 3, 7-trimethylxanthine
155. Bspbio_001921
156. Gtpl407
157. Kbiogr_002325
158. Kbioss_001781
159. 1,3,7-trimethyl-1,3,7-trihydropurine-2,6-dione
160. 5-26-13-00558 (beilstein Handbook Reference)
161. 95789-13-2
162. Mls001055341
163. Mls001056714
164. Mls001066409
165. Bidd:er0554
166. Bidd:gt0632
167. Divk1c_000730
168. Spectrum1500155
169. Cu-01000012617-3
170. Spbio_001222
171. Caffeine [orange Book]
172. Esgic Component Caffeine
173. Triad Component Caffeine
174. Megxp0_001350
175. Zinc1084
176. Caffeine [ep Monograph]
177. Dimenhydrinate Impurity C
178. Femcet Component Caffeine
179. Trezix Component Caffeine
180. 1,3,7-trimethyl-2,6-dioxo-1,2,3,6-tetrahydropurine
181. Anhydrous Caffeine [jan]
182. Caffeine [usp Monograph]
183. Component Of Dilone (salt/mix)
184. Dtxsid0020232
185. 1,7-trimethyl-2,6-dioxopurine
186. Acon1_000085
187. Anoquan Component Caffeine
188. Bdbm10849
189. Hms502e12
190. Kbio1_000730
191. Kbio2_001781
192. Kbio2_004349
193. Kbio2_006917
194. Kbio3_001141
195. Caffeine 1.0 Mg/ml In Methanol
196. Coffeinum [who-ip Latin]
197. Nsc5036
198. Cafergot Component Caffeine
199. Caffeine, Powder, Reagentplus(r)
200. Component Of Percodan (salt/mix)
201. Excedrin Component Caffeine
202. Fioricet Component Caffeine
203. Fiorinal Component Caffeine
204. Migergot Component Caffeine
205. Ninds_000730
206. Norgesic Component Caffeine
207. Wigraine Component Caffeine
208. Bio1_000473
209. Bio1_000962
210. Bio1_001451
211. Caffeine Component Of Esgic
212. Caffeine Component Of Triad
213. Dhc Plus Component Caffeine
214. Hms1920i09
215. Hms2091o11
216. Hms2232m13
217. Hms3260n17
218. Hms3372j18
219. Hms3435f10
220. Hms3715d13
221. Pharmakon1600-01500155
222. Invagesic Component Caffeine
223. Lanorinal Component Caffeine
224. Nodoz Caplets And Chewable Tablets
225. Caffeine 10 Microg/ml In Methanol
226. Caffeine Anhydrous [who-ip]
227. Caffeine Component Of Femcet
228. Cs-m0795
229. Caffeine Component Of Anoquan
230. Tox21_201685
231. Tox21_300010
232. Tox21_500228
233. Caffeine (1,3,7-trimethylxanthine)
234. Caffeine 100 Microg/ml In Methanol
235. Ccg-38825
236. Nsc755917
237. Orphengesic Component Caffeine
238. Pdsp1_001016
239. Pdsp1_001235
240. Pdsp2_001000
241. Pdsp2_001219
242. Stk177283
243. Caffeine Component Of Cafergot
244. Caffeine Component Of Dhc Plus
245. Caffeine Component Of Excedrin
246. Caffeine Component Of Fioricet
247. Caffeine Component Of Fiorinal
248. Caffeine Component Of Migergot
249. Caffeine Component Of Norgesic
250. Caffeine Component Of Wigraine
251. Propoxyphene Compound 65 (salt/mix)
252. Synalgos-dc Component Caffeine
253. Akos000121334
254. Caffeine Component Of Invagesic
255. Caffeine Component Of Lanorinal
256. 5-26-13-00558 (beilstein)
257. Bayer Select Headache Pain (salt/mix)
258. Caffeine, Anhydrous, 99%, Fcc, Fg
259. Db00201
260. Lp00228
261. Nsc-755917
262. Sdccgmls-0064595.p001
263. Sdccgmls-0064595.p002
264. Sdccgsbi-0050216.p005
265. Caffeine Component Of Orphengesic
266. Idi1_000730
267. Medigesic Plus Component Caffeine
268. Synalgos-dc-a Component Caffeine
269. 3,3,7-trimethyl-1h-purine-2,6-dione
270. Caffeine Component Of Synalgos-dc
271. Darvon Compound Component Caffeine
272. Invagesic Forte Component Caffeine
273. Ncgc00015208-01
274. Ncgc00015208-02
275. Ncgc00015208-03
276. Ncgc00015208-04
277. Ncgc00015208-05
278. Ncgc00015208-06
279. Ncgc00015208-07
280. Ncgc00015208-08
281. Ncgc00015208-10
282. Ncgc00015208-11
283. Ncgc00015208-12
284. Ncgc00015208-13
285. Ncgc00015208-14
286. Ncgc00015208-15
287. Ncgc00015208-16
288. Ncgc00015208-17
289. Ncgc00015208-18
290. Ncgc00015208-20
291. Ncgc00015208-29
292. Ncgc00090699-01
293. Ncgc00090699-02
294. Ncgc00090699-03
295. Ncgc00090699-04
296. Ncgc00090699-05
297. Ncgc00090699-06
298. Ncgc00090699-07
299. Ncgc00090699-08
300. Ncgc00090699-09
301. Ncgc00168808-01
302. Ncgc00168808-02
303. Ncgc00254057-01
304. Ncgc00259234-01
305. Ncgc00260913-01
306. Ac-12774
307. As-15340
308. Caffeine, Saj Special Grade, >=98.5%
309. Component Of P-a-c Compound (salt/mix)
310. Caffeine Component Of Synalgos-dc-a
311. Component Of A.s.a. Compound (salt/mix)
312. Orphengesic Forte Component Caffeine
313. Sbi-0050216.p004
314. Caffeine Component Of Medigesic Plus
315. Db-023002
316. Wln: T56 Bn Dn Fnvnvj B1 F1 H1
317. Caffeine Component Of Darvon Compound
318. Caffeine Component Of Invagesic Forte
319. Eu-0100228
320. Ft-0664195
321. N1379
322. N2379
323. Theophylline Impurity A [ep Impurity]
324. Caffeine Component Of Orphengesic Forte
325. Bim-0050216.0001
326. C07481
327. D00528
328. Dimenhydrinate Impurity C [ep Impurity]
329. Pentoxifylline Impurity F [ep Impurity]
330. Q60235
331. 1,3,7-trimethyl-3,7-dihydropurine-2,6-dione
332. 1h-purine-2, 3,7-dihydro-1,3,7-trimethyl-
333. Ab00051930-09
334. Ab00051930_10
335. Caffeine, Purum, Anhydrous, >=99.0% (hplc)
336. 3,7-dihydro-1,3,7-trimethyl-1h-purine (9ci)
337. Caffeine, Anhydrous, Tested According To Ph.eur.
338. L000155
339. 3,7-dihydro-1,3,7-trimethyl-1h-purin-2,6-dion
340. Caffeine, Sigma Reference Standard, Vial Of 250 Mg
341. Sr-01000075187-1
342. Sr-01000075187-4
343. Sr-01000075187-7
344. Sr-01000075187-8
345. Brd-k02404261-001-02-7
346. Brd-k02404261-001-03-5
347. Brd-k02404261-001-07-6
348. Caffeine, Certified Reference Material, Tracecert(r)
349. Caffeine, Meets Usp Testing Specifications, Anhydrous
350. Melting Point Standard 235-237c, Analytical Standard
351. 1,3,7-trimethyl-3,7-dihydro-1h-purine-2,6-dione #
352. Caffeine, British Pharmacopoeia (bp) Reference Standard
353. Caffeine, European Pharmacopoeia (ep) Reference Standard
354. F3371-0262
355. Theophylline Monohydrate Impurity A [ep Impurity]
356. Z112207564
357. Caffeine 2000 Microg/ml In Water:methanol (81:19 G/g)
358. 07e4fb58-fd79-4175-8e3d-05bf96954522
359. 3,7-dihydro-1,3,7-trimethyl-1h-purin-2,6-dion (coffein)
360. Caffeine Solution, Analytical Standard, 1.0 Mg/ml In Methanol
361. Caffeine, United States Pharmacopeia (usp) Reference Standard
362. Caffeine, Pharmaceutical Secondary Standard; Certified Reference Material
363. Caffeine For System Suitability, European Pharmacopoeia (ep) Reference Standard
364. Caffeine Melting Point Standard, United States Pharmacopeia (usp) Reference Standard
365. Caffeine Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
366. 114303-55-8
367. Caffeine Melting Point Standard, Pharmaceutical Secondary Standard; Certified Reference Material
368. Caffeine, Pharmagrade, Ep, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production
369. Mettler-toledo Calibration Substance Me 18872, Caffeine, Analytical Standard, For The Calibration Of The Thermosystem 900, Traceable To Primary Standards (lgc)
1. Caffeine Mixture With Ergotamine
2. Ergotamine Mixture With Caffeine
3. Cafergamine
| Molecular Weight | 194.19 g/mol |
|---|---|
| Molecular Formula | C8H10N4O2 |
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 194.08037557 g/mol |
| Monoisotopic Mass | 194.08037557 g/mol |
| Topological Polar Surface Area | 58.4 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 293 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Cafcit |
| PubMed Health | Caffeine Citrate (By mouth) |
| Drug Classes | Stimulant, Respiratory |
| Active Ingredient | Caffeine citrate |
| Dosage Form | Solution |
| Route | Intravenous; Oral |
| Strength | eq 30mg base/3ml (eq 10mg base/ml) |
| Market Status | Prescription |
| Company | Eurohlth Intl |
| 2 of 6 | |
|---|---|
| Drug Name | Lanorinal |
| PubMed Health | Aspirin/Caffeine/Dihydrocodeine (By mouth) |
| Drug Classes | Analgesic, Opioid/Salicylate, Aspirin Combination |
| Active Ingredient | butalbital; caffeine; Aspirin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 50mg; 325mg; 40mg |
| Market Status | Prescription |
| Company | Lannett |
| 3 of 6 | |
|---|---|
| Drug Name | Synalgos-dc |
| Active Ingredient | dihydrocodeine bitartrate; caffeine; Aspirin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 30mg; 356.4mg; 16mg |
| Market Status | Prescription |
| Company | Caraco |
| 4 of 6 | |
|---|---|
| Drug Name | Cafcit |
| PubMed Health | Caffeine Citrate (By mouth) |
| Drug Classes | Stimulant, Respiratory |
| Active Ingredient | Caffeine citrate |
| Dosage Form | Solution |
| Route | Intravenous; Oral |
| Strength | eq 30mg base/3ml (eq 10mg base/ml) |
| Market Status | Prescription |
| Company | Eurohlth Intl |
| 5 of 6 | |
|---|---|
| Drug Name | Lanorinal |
| PubMed Health | Aspirin/Caffeine/Dihydrocodeine (By mouth) |
| Drug Classes | Analgesic, Opioid/Salicylate, Aspirin Combination |
| Active Ingredient | butalbital; caffeine; Aspirin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 50mg; 325mg; 40mg |
| Market Status | Prescription |
| Company | Lannett |
| 6 of 6 | |
|---|---|
| Drug Name | Synalgos-dc |
| Active Ingredient | dihydrocodeine bitartrate; caffeine; Aspirin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 30mg; 356.4mg; 16mg |
| Market Status | Prescription |
| Company | Caraco |
Central Nervous System Stimulants; Phosphodiesterase Inhibitors; Purinergic P1 Receptor Antagonists
National Library of Medicine's Medical Subject Headings. Caffeine. Online file (MeSH, 2014). Available from, as of January 30, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Caffeine is used orally as a mild CNS stimulant to aid in staying awake and to restore mental alertness in fatigued patients.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2567
Apnea of prematurity. Caffeine citrate is used iv or orally in the short-term (10-12 days) treatment of apnea of prematurity in neonates who are between 28 and less than 33 weeks of gestational age. Caffeine is designated an orphan drug by the US Food and Drug Administration (FDA) for use in apnea in premature neonates.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2567
Caffeine is used in combination with ergotamine tartrate to abort vascular headaches such as migraine and cluster headaches (histamine cephalalgia).
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2567
For more Therapeutic Uses (Complete) data for CAFFEINE (11 total), please visit the HSDB record page.
Because it has been suggested that caffeine may promote gastric ulceration, the drug should be used cautiously in patients with a history of peptic ulcer. Because of its suspected arrhythmogenic potential, it is generally recommended that caffeine be avoided in patients with symptomatic cardiac arrhythmias and/or palpitations and during the first several days to weeks after an acute myocardial infarction
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2568
Prior to initiation of caffeine citrate therapy, baseline serum concentrations of caffeine should be measured in neonates previously treated with theophylline, since preterm neonates metabolize theophylline to caffeine. Similarly, baseline serum concentrations of caffeine should be measured in infants born to mothers who consumed caffeine prior to delivery since caffeine readily crosses the placenta. Serious toxicity has been reported when serum caffeine concentrations exceed 50 ug/mL. /Caffeine citrate/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2568
In clinical trials reported in the literature, cases of hypoglycemia and hyperglycemia have been reported in patients receiving caffeine; therefore, blood glucose concentration may need to be monitored periodically in neonates receiving caffeine citrate. /Caffeine citrate/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2568
During the placebo-controlled trial of caffeine citrate establishing efficacy in the US for apnea of prematurity, 6 cases of necrotizing enterocolitis developed among the 85 neonates studied, 3 cases of which were fatal. Five of the 6 neonates had been randomized to treatment with or had been exposed to caffeine citrate. Reports in the literature have raised the possibility of an association betwen the use of methylxanthines and the development of necrotizing enterocolitis, although a causal relationship between methylxanthine use and the development of necrotizing enterocolitis have not been established. Therefore, as with all premature neonates, patients being treated with caffeine citrate should be monitored carefully for the development of necrotizing enterocolitis. /Caffeine citrate/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2568
For more Drug Warnings (Complete) data for CAFFEINE (25 total), please visit the HSDB record page.
In adults, iv doses of 57 mg/kg and oral doses of 18-50 g have been fatal.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2569
In one 5-year-old patient, death occurred following oral ingestion of approximately 3 g of caffeine.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2569
Caffeine is indicated for the short term treatment of apnea of prematurity in infants and off label for the prevention and treatment of bronchopulmonary dysplasia caused by premature birth. In addition, it is indicated in combination with sodium benzoate to treat respiratory depression resulting from an overdose with CNS depressant drugs. Caffeine has a broad range of over the counter uses, and is found in energy supplements, athletic enhancement products, pain relief products, as well as cosmetic products.
FDA Label
Caffeine stimulates the central nervous system (CNS), heightening alertness, and sometimes causing restlessness and agitation. It relaxes smooth muscle, stimulates the contraction of cardiac muscle, and enhances athletic performance. Caffeine promotes gastric acid secretion and increases gastrointestinal motility. It is often combined in products with analgesics and ergot alkaloids, relieving the symptoms of migraine and other types of headaches. Finally, caffeine acts as a mild diuretic.
Phosphodiesterase Inhibitors
Compounds which inhibit or antagonize the biosynthesis or actions of phosphodiesterases. (See all compounds classified as Phosphodiesterase Inhibitors.)
Purinergic P1 Receptor Antagonists
Compounds that bind to and block the stimulation of PURINERGIC P1 RECEPTORS. (See all compounds classified as Purinergic P1 Receptor Antagonists.)
Central Nervous System Stimulants
A loosely defined group of drugs that tend to increase behavioral alertness, agitation, or excitation. They work by a variety of mechanisms, but usually not by direct excitation of neurons. The many drugs that have such actions as side effects to their main therapeutic use are not included here. (See all compounds classified as Central Nervous System Stimulants.)
N06BC01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D11 - Other dermatological preparations
D11A - Other dermatological preparations
D11AX - Other dermatologicals
D11AX26 - Caffeine
N - Nervous system
N06 - Psychoanaleptics
N06B - Psychostimulants, agents used for adhd and nootropics
N06BC - Xanthine derivatives
N06BC01 - Caffeine
Absorption
Caffeine is rapidly absorbed after oral or parenteral administration, reaching peak plasma concentration within 30 minutes to 2 hours after administration. After oral administration, onset of action takes place within 45 to 1 hour. Food may delay caffeine absorption. The peak plasma level for caffeine ranges from 6-10mg/L. The absolute bioavailability is unavailable in neonates, but reaches about 100% in adults.
Route of Elimination
The major metabolites of caffeine can be found excreted in the urine. About 0.5% to 2% of a caffeine dose is found excreted in urine, as it because it is heavily absorbed in the renal tubules.
Volume of Distribution
Caffeine has the ability to rapidly cross the blood-brain barrier. It is water and fat soluble and distributes throughout the body. Caffeine concentrations in the cerebrospinal fluid of preterm newborns are similar to the concentrations found in the plasma. The mean volume of distribution of caffeine in infants is 0.8-0.9 L/kg and 0.6 L/kg in the adult population.
Clearance
The clearance of caffeine varies, but on average, is about 0.078L/kg/h (1.3mL/min/kg).
World-wide, many fetuses and infants are exposed to methylxanthines via maternal consumption of coffee and other beverages containing these substances. Methylxanthines (caffeine, theophylline and aminophylline) are also commonly used as a medication for apnea of prematurity. ... Methylxanthines readily passes the placenta barrier and enters all tissues and thus may affect the fetus/newborn at any time during pregnancy or postnatal life, given that the effector systems are mature. ...
PMID:20859804 Aden U; Handb Exp Pharmacol (200): 373-89 (2011)
Caffeine and citrated caffeine are well absorbed following oral administration. Absorption of caffeine following oral administration may be more rapid than that following IM injection of caffeine and sodium benzoate. Absorption following rectal administration of caffeine in suppositories may be slow and erratic. ... Following oral administration of 100 mg of caffeine (as coffee), peak plasma concentrations of about 1.5-1.8 ug/mL are reached after 50-75 minutes.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2569
After oral administration of 10 mg caffeine base/kg to preterm neonates, the peak plasma concentration for caffeine ranged from 6-10 mg/L and the mean time to reach peak concentration /Tmax/ ranged from 30 minutes to 2 hours. The /Tmax/ was not affected by formula feeding.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2569
Caffeine is rapidly distributed into body tissues, readily crossing the placenta and blood-brain barrier. Caffeine concentration in the CSF fluid of preterm neonates approximates the plasma concentration. The mean volume of distribution of caffeine in infants (0.8-0.9 L/kg) is slightly higher than that in adults (0.6 L/kg). ... Caffeine has been shown to distribute into milk in a milk-to-serum concentration ratio of 0.5-0.76.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2569
For more Absorption, Distribution and Excretion (Complete) data for CAFFEINE (11 total), please visit the HSDB record page.
Caffeine metabolism occurs mainly in the liver via the cytochrome CYP1A2 enzyme. The products of caffeine metabolism include paraxanthine, theobromine, and theophylline. The first step of caffeine metabolism is demethylation, yielding paraxanthine (a major metabolite), followed by theobromine, and theophylline, which are both minor metabolites. They are then excreted in urine as urates after additional metabolism. The enzymes xanthine oxidase and N-acetyltransferase 2 (NAT2) also participate in the metabolism of caffeine.
Caffeine is metabolized by the cytochrome P-450 (CYP) enzyme system, principally by isoenzyme 1A2. Therefore, caffeine has the potential to interact with drugs that are metabolized by CYP1A2 or with drugs that induce or inhibit this isoenzyme.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2569
In adults, the drug is rapidly metabolized in the liver to 1-methyluric acid, 1-methylxanthine, and 7-methylxanthine.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2569
Interconversion between caffeine and theophylline has been reported in preterm neonates...
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2569
In-vivo and in-vitro experiments showed a progressive increase in the activity of the hepatic microsomal enzymes that metabolize caffeine during neonatal development. In beagle puppies, change in caffeine clearance was determined by the rate of maturation of caffeine-7-demethylase. Caffeine is eliminated in animals by biotransformation in the liver to dimethylxanthines, dimethyl- and monomethyluric acids and uracil derivatives; important quantitative differences have been demonstrated in the formation and elimination of metabolites in rats, mice and Chinese hamsters. These differences are even more important in monkeys, where caffeine is almost completely metabolized to theophylline. ... Some species-dependent metabolites have been identified. Trimethylallantoin was first reported in rats. A ... derivative of paraxanthine was found in mice and identified as the 3-beta-D-glucuronide of paraxanthine. Methylated ureas and sulfur-containing derivatives found in urine in trace amounts are produced by the intestinal flora. In contrast, the acetylated uracil derivative, 5-acetylamino-6-formylamino-3-methyluracil, one of the most important caffeine metabolites in humans, has not been identified in rodents or other animal species. Other uracil derivatives produced from caffeine, theobromine and paraxanthine in rats were found in human urine. In rats, the hepatic demethylation of caffeine shows an age-related decline, resulting in a greatly increased elimination half-time in older adult rats.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 322 (1991)
Caffeine metabolism is qualitatively relatively similar in animals and humans ... . The main metabolic pathways are: demethylation and hydroxylation of the 8-position leading to the formation of the respective uracil and uric acid derivatives. There are, however, some quantitative differences in the metabolic profile. Humans are characterized by the importance of 3-methyl demethylation leading to the formation of paraxanthine and especially metabolites thereof through subsequent metabolic steps. The main urinary metabolites in humans are 1-methyluric acid, 1-methylxanthine, 5-acetylamino-6-formylamino-3- methyluracil (not found in rats and mice), 1,7-dimethyluric acid and paraxanthin. In rats and mice, the metabolism of caffeine is predominantly via theobromine and theophylline. The main urinary metabolites are 1,3-dimethyluracil, paraxanthine, trimethyluric acid, theophylline, and theobromine. Caffeine metabolism decreases during pregnancy, resulting in higher serum concentrations.
OECD SIDS for Caffeine (CAS 58-08-2); Available from, as of January 9, 2014: https://www.inchem.org/documents/sids/sids/CAFEINE.pdf
Caffeine has known human metabolites that include 1,3,7-Trimethyluric acid, Theobromine, paraxanthine, and theophylline.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
In an average-sized adult or child above the age of 9, the half-life of caffeine is approximately 5 hours. Various characteristics and conditions can alter caffeine half-life. It can be reduced by up to 50% in smokers. Pregnant women show an increased half-life of 15 hours or higher, especially in the third trimester. The half-life in newborns is prolonged to about 8 hours at full-term and 100 hours in premature infants, likely due to reduced ability to metabolize it. Liver disease or drugs that inhibit CYP1A2 can increase caffeine half-life.
Elimination 1/2 life in adults = 2.5-4.5 hours; [Reference #1]
Caffeine has a plasma half-life (t1/2) of 3 to 5 hours in adults. In one study, when administered to pregnant women prior to delivery, caffeine had a prolonged mean half-life of 80 hours in neonates after delivery.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2569
Mean half-life /T 1/2/ and fraction excreted unchanged in urine of caffeine in infants have been shown to be inversely related to gestational/postconceptual age. In neonates, the /T 1/2/ is approximately 3-4 days...
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2569
The half-time for caffeine is 0.7-1.0 hr in rats and mice, 1-1.6 hr in rabbits, 3-5 hr in monkeys, 4-4.3 hr in dogs and 11-12 hr in baboons.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 321 (1991)
/The authors/ studied 17 preterm infants receiving caffeine, and measured their plasma levels of caffeine and the theophylline metabolite by high-pressure liquid chromatography. The half-life was calculated by computer analysis using the least-square method. The mean gestational age of our patients was 29.7 +/- 1.9 weeks (mean +/- SD) and they were studied at 20.7 +/- 6.6 days (mean +/- SD) postnatal age. The caffeine half-life was 52.03 +/- 23.87 hr (means +/- SD) and the theophylline half-life was 77.04 +/- 65.01 hr (mean +/- SD).
PMID:2714159 Pearlman SA et al; Dev Pharmacol Ther 12 (2): 65-9 (1989)
The mechanism of action of caffeine is complex, as it impacts several body systems, which are listed below. The effects as they relate to various body systems are described as follows: **General and cellular actions** Caffeine exerts several actions on cells, but the clinical relevance is poorly understood. One probable mechanism is the inhibition of nucleotide phosphodiesterase enzymes, adenosine receptors, regulation of calcium handling in cells, and participates in adenosine receptor antagonism. Phosphodiesterase enzymes regulate cell function via actions on second messengers cAMP and cGMP. This causes lipolysis through activation of hormone-sensitive lipases, releasing fatty acids and glycerol. **Respiratory** The exact mechanism of action of caffeine in treating apnea related to prematurity is unknown, however, there are several proposed mechanisms, including respiratory center stimulation in the central nervous system, a reduced threshold to hypercapnia with increased response, and increased consumption of oxygen, among others. The blocking of the adenosine receptors enhances respiratory drive via an increase in brain medullary response to carbon dioxide, stimulating ventilation and respiratory drive, while increasing contractility of the diaphragm. **Central nervous system** Caffeine demonstrates antagonism of all 4 adenosine receptor subtypes (A1, A2a, A2b, A3) in the central nervous system. Caffeine's effects on alertness and combatting drowsiness are specifically related to the antagonism of the A2a receptor. **Renal system** Caffeine has diuretic effects due to is stimulatory effects on renal blood flow, increase in glomerular filtration, and increase in sodium excretion. **Cardiovascular system** Adenosine receptor antagonism at the A1 receptor by caffeine stimulates inotropic effects in the heart. Blocking of adenosine receptors promotes catecholamine release, leading to stimulatory effects occurring in the heart and the rest of the body. In the blood vessels, caffeine exerts direct antagonism of adenosine receptors, causing vasodilation. It stimulates the endothelial cells in the blood vessel wall to release nitric oxide, potentiating blood vessel relaxation. Catecholamine release, however, antagonizes this and exerts inotropic and chronotropic effects on the heart, ultimately leading to vasoconstriction. Finally, caffeine is shown to raise systolic blood pressure measurements by 5 to 10 mmHg when it is not taken regularly, versus no effect in those who consume it regularly. The vasoconstricting effects of caffeine are beneficial in migraines and other types of headache, which are normally caused by vasodilation in the brain.
Caffeine competitively inhibits phosphodiesterase, the enzyme that degrades cyclic 3',5'-adenosine monophosphate (AMP). Increased levels of intracellular cyclic AMP mediate most of caffeine's pharmacologic actions.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2569
Caffeine stimulates all levels of the CNS... Caffeine's cortical effects are milder and of shorter duration than those of amphetamines. In slightly larger doses, caffeine stimulates medullary, vagal, vasomotor, and respiratory centers, promoting bradycardia, vasoconstriction, and increased respiratory rate.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2569
Caffeine constricts cerebral vasculature. In contrast, the drug directly dilates peripheral blood vessels...
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2569

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
15
PharmaCompass offers a list of Caffeine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Caffeine manufacturer or Caffeine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Caffeine manufacturer or Caffeine supplier.
PharmaCompass also assists you with knowing the Caffeine API Price utilized in the formulation of products. Caffeine API Price is not always fixed or binding as the Caffeine Price is obtained through a variety of data sources. The Caffeine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Caffeine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Caffeine, including repackagers and relabelers. The FDA regulates Caffeine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Caffeine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Caffeine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Caffeine supplier is an individual or a company that provides Caffeine active pharmaceutical ingredient (API) or Caffeine finished formulations upon request. The Caffeine suppliers may include Caffeine API manufacturers, exporters, distributors and traders.
click here to find a list of Caffeine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Caffeine DMF (Drug Master File) is a document detailing the whole manufacturing process of Caffeine active pharmaceutical ingredient (API) in detail. Different forms of Caffeine DMFs exist exist since differing nations have different regulations, such as Caffeine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Caffeine DMF submitted to regulatory agencies in the US is known as a USDMF. Caffeine USDMF includes data on Caffeine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Caffeine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Caffeine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Caffeine Drug Master File in Japan (Caffeine JDMF) empowers Caffeine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Caffeine JDMF during the approval evaluation for pharmaceutical products. At the time of Caffeine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Caffeine suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Caffeine Drug Master File in Korea (Caffeine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Caffeine. The MFDS reviews the Caffeine KDMF as part of the drug registration process and uses the information provided in the Caffeine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Caffeine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Caffeine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Caffeine suppliers with KDMF on PharmaCompass.
A Caffeine CEP of the European Pharmacopoeia monograph is often referred to as a Caffeine Certificate of Suitability (COS). The purpose of a Caffeine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Caffeine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Caffeine to their clients by showing that a Caffeine CEP has been issued for it. The manufacturer submits a Caffeine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Caffeine CEP holder for the record. Additionally, the data presented in the Caffeine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Caffeine DMF.
A Caffeine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Caffeine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Caffeine suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Caffeine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Caffeine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Caffeine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Caffeine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Caffeine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Caffeine suppliers with NDC on PharmaCompass.
Caffeine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Caffeine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Caffeine GMP manufacturer or Caffeine GMP API supplier for your needs.
A Caffeine CoA (Certificate of Analysis) is a formal document that attests to Caffeine's compliance with Caffeine specifications and serves as a tool for batch-level quality control.
Caffeine CoA mostly includes findings from lab analyses of a specific batch. For each Caffeine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Caffeine may be tested according to a variety of international standards, such as European Pharmacopoeia (Caffeine EP), Caffeine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Caffeine USP).
