

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
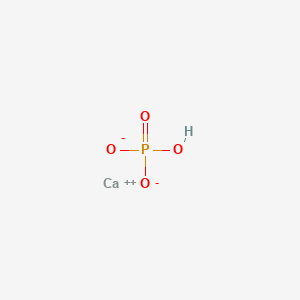

1. Anhydrous Dibasic Calcium Phosphate
2. Calcium Phosphate (cahpo4)
3. Calcium Phosphate, Dibasic, Anhydrous
4. Dibasic Calcium Phosphate, Anhydrous
5. Monetite
1. 7757-93-9
2. Calcium Phosphate Dibasic
3. Dicalcium Phosphate
4. Calcium Phosphate, Dibasic
5. Dibasic Calcium Phosphate
6. Phosphoric Acid, Calcium Salt (1:1)
7. Secondary Calcium Phosphate
8. Calcium Phosphate (1:1)
9. Dicalcium Orthophosphate
10. Calcium Hydrogen Orthophosphate
11. Monocalcium Acid Phosphate
12. Dicalcium Phosphate Anhydrous
13. Calcium Monohydrogen Phosphate
14. Calciumphosphate,dibasic
15. Calcium Phosphate Dibasic Anhydrous
16. Anhydrous Dibasic Calcium Phosphate
17. Calcium Phosphate,dibasic
18. Mfcd00010909
19. Calcium Phosphate (dibasic)
20. Chebi:32596
21. Ins-341(ii)
22. L11k75p92j
23. Calcium Phosphate, Dibasic, Anhydrous
24. E-341(ii)
25. Biofos
26. Fujicalin S
27. Dicafos An
28. 7557-93-9
29. Anhydrous Emcompress
30. Ipifosc 20
31. A-tab
32. D-glucopyranosiduronicacid, 1-(2,4-dichlorophenyl)ethyl
33. Calcium Acid Phosphate
34. Calcium Dibasic Phosphate
35. Calcium Secondary Phosphate
36. Calcium Phosphate (cahpo4)
37. Monohydrogen Calcium Phosphate
38. Ccris 1337
39. Hsdb 992
40. Cahpo4
41. Calcium Phosphate, Dibasic [jan]
42. Einecs 231-826-1
43. Calcium Hydrogenorthophosphate
44. Calcium Phosphate, Dibasic, Dental Grade
45. Unii-l11k75p92j
46. Calstar (tn)
47. Calcium Monohydrogen Phosphate Anhydrous
48. Calcii Hydrogenophosphas
49. Calcium;hydrogen Phosphate
50. Ec 231-826-1
51. Calcium Monohydrogenphosphate
52. Dicalcium Phosphate, Anhydrous
53. Calcium (as Phosphate,dibasic)
54. Ins No.341(ii)
55. Chembl2107567
56. Dtxsid20872529
57. Dicalcium Phosphate [inci]
58. Phosphoric Acid Calcium Salt
59. Calcium Hydrogen Monophosphate
60. Calcium Hydrogen Phosphate, Anhydrous
61. Akos015965054
62. Calcium Hydrogenorthophosphate Anhydrous
63. Calcium Phosphate, Dibasic [mi]
64. Calcium Phosphate,dibasic [vandf]
65. Calcium Hydrogenorthophosphate, With A Fluorine Content Of Less Than 0,005 Per Cent By Weight On The Dry Anhydrous Product
66. Calcium Phosphate, Dibasic [hsdb]
67. Dibasic Calcium Phosphate Anhydrous
68. Calcium Hydrogen Phosphate Anhydrous
69. Calcium Phosphate Dibasic [who-dd]
70. Calcium Phosphate, Dibasic (99.95%-ca)
71. Phosphorus (as Dicalcium Phosphate)
72. Calcium Phosphate (1:1) [who-ip]
73. Calcium Phosphate Dibasic, 98.0-105.0%
74. Calcium Phosphate, Dibasic Anhydrous
75. Dibasic Calcium Phosphate, Anhydrous (jan)
76. Calcium Phosphate Dibasic, Puriss., 98.0%
77. Ft-0623397
78. Calcium (calcium Phosphate (dibasic))
79. Calcium Hydrogen Phosphate (anhydrous)
80. Calcii Hydrogenophosphas [who-ip Latin]
81. Calcium (as Phosphate,dibasic) [vandf]
82. D03302
83. Anhydrous Dibasic Calcium Phosphate [jan]
84. Calcium Hydrogen Phosphate [ep Monograph]
85. Calcium Phosphate Dibasic Anhydrous, Usp Fcc Pe
86. Calcium Phosphate Dibasic, Usp, 98.0-103.0%
87. Calcium Phosphate, Dibasic, Anhydrous [ii]
88. Q414619
89. Phosphorus (as Dicalcium Phosphate) [vandf]
90. Calcium Hydrogen Phosphate, Anhydrous [who-ip]
91. Anhydrous Dibasic Calcium Phosphate [usp Monograph]
92. Dibasic Calcium Phosphate, Meets Usp Testing Specifications
93. Phosphorus (as Calcium Phosphate,dibasic) [vandf]
94. Calcium Phosphate Dibasic, Puriss. P.a., Anhydrous, >98.5% (kt)
| Molecular Weight | 136.06 g/mol |
|---|---|
| Molecular Formula | CaHO4P |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 135.9238364 g/mol |
| Monoisotopic Mass | 135.9238364 g/mol |
| Topological Polar Surface Area | 83.4 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 46.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
USES--EXCELLENT SOURCE OF CALCIUM & PHOSPHORUS DURING PREGNANCY, LACTATION, OR MILD TO MODERATE HYPOCALCEMIA CHARACTERIZED BY LOW DEGREE OF TETANY. ... IF TETANY IS SEVERE, IV CALCIUM MEDICATION IS ADMIN. ... DOSE--1 TO 5 G/DAY IN 2 OR MORE DIVIDED DOSES.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 770
IT IS NOT MARKETED IN SINGLE ENTITY FORM, SO THAT DOSE OF OTHER INGREDIENTS MAY HAVE TO BE CONSIDERED IN ARRIVING @ SUITABLE SCHEDULE. CALCIUM IS BETTER ABSORBED IF TAKEN IN SMALL DOSES @ FREQUENT INTERVALS.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 770
MEDICATION (VET): FOR PREVENTION & TREATMENT OF RICKETS ALONG WITH VITAMIN D THERAPY, & AS NUTRIENT SOURCE OF CALCIUM & PHOSPHORUS IN FEEDSTUFFS. ... OPTIMUM CALCIUM:PHOSPHORUS RATIO IN DIET MUST ALSO BE MAINTAINED.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 71
DIBASIC CALCIUM PHOSPHATE, NATIONAL FORMULARY ... /IS AMONG CMPD/ CLASSIFIED AS NONSYSTEMIC GASTRIC ANTACIDS, BUT THEIR EFFICACY IS LOW & THEY ARE SELDOM EMPLOYED.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 967
Medication (VET): Has been used as a dietary supplement, and as an antacid.
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 256
BECAUSE OF PHOSPHATE CONTENT, IT IS CONTRAINDICATED IN /PERSONS WITH/ HYPOPARATHYROIDISM.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 770
... ITS ELEMENTS ARE SLOWLY ABSORBED FROM SMALL INTESTINE.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 71
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40121
Submission : 2024-06-23
Status : Active
Type : IV


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 31193
Submission : 2016-12-03
Status : Active
Type : IV


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 31307
Submission : 2017-01-06
Status : Active
Type : IV


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37802
Submission : 2022-12-12
Status : Active
Type : IV


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 12692
Submission : 1997-10-08
Status : Active
Type : IV






 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 12692
Submission : 1997-10-08
Status : Active
Type : IV

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 31307
Submission : 2017-01-06
Status : Active
Type : IV

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 31193
Submission : 2016-12-03
Status : Active
Type : IV

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37802
Submission : 2022-12-12
Status : Active
Type : IV

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40121
Submission : 2024-06-23
Status : Active
Type : IV

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS
NDC Package Code : 55570-180
Start Marketing Date : 2008-02-27
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
