
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
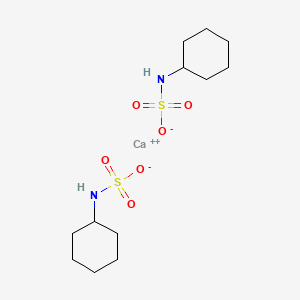

1. Cyclamate
2. Cyclamate Calcium (2:1) Salt
3. Cyclamate, Calcium
4. Cyclamate, Calcium (2:1) Salt, Dihydrate
5. Cyclamate, Potassium
6. Cyclamate, Sodium
7. Cyclamate, Sodium Salt
8. Cyclamates
9. Cyclamic Acid
10. Potassium Cyclamate
11. Sodium Cyclamate
1. 139-06-0
2. Cyclamate Calcium
3. Cyclan
4. Calcium Bis(cyclohexylsulphamate)
5. Calcium Cyclohexanesulfamate
6. Calcium Cyclamate(1:2)
7. Calcium;n-cyclohexylsulfamate
8. X12lmj1wyl
9. Calcium Cyclamate Anhydrous
10. Dietil
11. Cylan
12. Sucaryl Calcium
13. Cyclamate, Calcium Salt
14. Kalziumzyklamate
15. Kalziumzyklamate [german]
16. Calcium Cyclohexylsulphamate
17. Calcium Cyclohexane Sulphamate
18. Ccris 186
19. Hsdb 823
20. Calcium Cyclohexylsulfamate (1:2)
21. Einecs 205-349-4
22. Unii-x12lmj1wyl
23. Ai3-52126
24. Cyclohexanesulfamic Acid, Calcium Salt (2:1)
25. Sulfamic Acid, Cyclohexyl-, Calcium Salt (2:1)
26. Calciumcyclohexylsulfamat
27. Calcium Cyclohexylsulfamate
28. Calcium Cyclamate [mi]
29. Schembl1532404
30. Calcium Cyclamate [hsdb]
31. Calcium Cyclamate [inci]
32. Chembl2104112
33. Dtxsid9057892
34. Cyclamate Calcium Anhydrous
35. Calcium Cyclamate, Anhydrous
36. Calcium N-cyclohexylsulphamate
37. Cyclohexylsulfamic Acid Calcium Salt
38. Db-042461
39. Ft-0631808
40. D02444
41. Sulfamic Acid, N-cyclohexyl-, Calcium Salt (2:1)
42. Q27293257
| Molecular Weight | 396.5 g/mol |
|---|---|
| Molecular Formula | C12H24CaN2O6S2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 396.0701697 g/mol |
| Monoisotopic Mass | 396.0701697 g/mol |
| Topological Polar Surface Area | 155 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 187 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
2. 2= SLIGHTLY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 5-15 G/KG, BETWEEN 1 PINT & 1 QUART FOR 70 KG PERSON (150 LB).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-406
Sweetening Agents
Substances that sweeten food, beverages, medications, etc., such as sugar, saccharine or other low-calorie synthetic products. (From Random House Unabridged Dictionary, 2d ed) (See all compounds classified as Sweetening Agents.)
...PREVIOUSLY THOUGHT TO BE METABOLICALLY STABLE. WHEN GIVEN ORALLY TO GUINEA PIGS, RABBITS, RATS, & HUMANS ON CYCLAMATE-FREE DIET IT IS EXCRETED LARGELY UNCHANGED. /CYCLAMATE/
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 449
...CYCLOHEXYLAMINE FORMED IN INTESTINE IS ALMOST COMPLETELY ABSORBED & EXCRETED IN URINE, IN COMPARISON TO CYCLAMATE, WHICH IS ABSORBED TO AN EXTENT OF ONLY 20-40%. /CYCLAMATE/
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 264
...(14)C-CYCLAMATE, GIVEN IV TO WOMEN UNDERGOING THERAPEUTIC ABORTION, BY ABDOMINAL HYSTERECTOMY DURING EARLY PREGNANCY, CROSSED PLACENTA & DISPERSED IN FETAL TISSUES, PARTICULARLY IN LIVER, SPLEEN, PANCREAS, & KIDNEYS. ...MATERNAL BLOOD LEVELS...FELL RAPIDLY...INDICATING VERY RAPID ELIMINATION... /CYCLAMATE/
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 150
MAX DAILY EXCRETION OF CYCLOHEXYLAMINE...RANGED FROM 0.1 TO 0.9% OF DAILY CYCLAMATE INTAKE (5 G); URINARY OUTPUT OF CYCLOHEXYLAMINE WAS ERRATIC & CONTINUED FOR 3-4 DAYS AFTER ADMIN HAD CEASED. /CYCLAMATE/
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 265
For more Absorption, Distribution and Excretion (Complete) data for CALCIUM CYCLAMATE (6 total), please visit the HSDB record page.
/CALCIUM/ CYCLAMATE LABELLED WITH (35)S OR (14)C IS EXCRETED BY MAN & LABORATORY ANIMALS ALMOST ENTIRELY UNCHANGED, IN URINE & FECES, ALTHOUGH TRACES OF...CYCLOHEXYLAMINE (0.7% DOSE) HAVE BEEN FOUND IN URINE OF HUMANS & DOGS DOSED ORALLY...
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 165
...BIOTRANSFORMATION IN RATS...IS BROUGHT ABOUT BY GUT MICROFLORA. ...GUT MICROFLORA IN MAN & IN ANIMALS ACQUIRE CAPACITY TO METABOLIZE CYCLAMATE INTO CYCLOHEXYLAMINE, WHEN CYCLAMATE IS ADMIN CHRONICALLY. /CYCLAMATE/
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 264
GUT MICRO-ORGANISMS, WHICH BECOME CYCLAMATE "CONVERTERS" ARE CLOSTRIDIA IN RATS, ENTEROBACTERIA IN RABBITS, & ENTEROCOCCI IN MAN. /CYCLAMATE/
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 347
...2 GROUPS OF WEANLING RATS WERE FED FOR 8 MO...WITH CHOW DIET OR...DIET CONTAINING 0.1% CA CYCLAMATE...THEN A SINGLE DOSE OF (14)C-CYCLAMATE BY STOMACH TUBE. ...7/11 CYCLAMATE-FED RATS CONVERTED CYCLAMATE INTO CYCLOHEXYLAMINE, TO...12-25% OF TOTAL (14)C IN URINE. 2 OF THE URINES CONTAINED TRACES OF DICYCLOHEXYLAMINE...
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 264
WHEN GUINEA PIGS, RABBITS, RATS, & HUMANS WERE PLACED ON A DIET CONTAINING CALCIUM CYCLAMATE THEY DEVELOPED THE ABILITY TO CONVERT ORALLY ADMIN CYCLAMATE INTO CYCLOHEXYLAMINE & THE METABOLITES OF THE LATTER.
RENWICK ET AL; BIOCHEM J 129(4) 869 (1972)
...IV DOSE OF 14(C)-CYCLAMATE TO PREGNANT RAT...T/2 OF CYCLAMATE WAS ABOUT 7 HR. /CYCLAMATE/
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 150
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
52
PharmaCompass offers a list of Calcium Cyclamate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Calcium Cyclamate manufacturer or Calcium Cyclamate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Calcium Cyclamate manufacturer or Calcium Cyclamate supplier.
PharmaCompass also assists you with knowing the Calcium Cyclamate API Price utilized in the formulation of products. Calcium Cyclamate API Price is not always fixed or binding as the Calcium Cyclamate Price is obtained through a variety of data sources. The Calcium Cyclamate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Calcium Cyclamate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Calcium Cyclamate, including repackagers and relabelers. The FDA regulates Calcium Cyclamate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Calcium Cyclamate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Calcium Cyclamate supplier is an individual or a company that provides Calcium Cyclamate active pharmaceutical ingredient (API) or Calcium Cyclamate finished formulations upon request. The Calcium Cyclamate suppliers may include Calcium Cyclamate API manufacturers, exporters, distributors and traders.
Calcium Cyclamate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Calcium Cyclamate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Calcium Cyclamate GMP manufacturer or Calcium Cyclamate GMP API supplier for your needs.
A Calcium Cyclamate CoA (Certificate of Analysis) is a formal document that attests to Calcium Cyclamate's compliance with Calcium Cyclamate specifications and serves as a tool for batch-level quality control.
Calcium Cyclamate CoA mostly includes findings from lab analyses of a specific batch. For each Calcium Cyclamate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Calcium Cyclamate may be tested according to a variety of international standards, such as European Pharmacopoeia (Calcium Cyclamate EP), Calcium Cyclamate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Calcium Cyclamate USP).
