
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
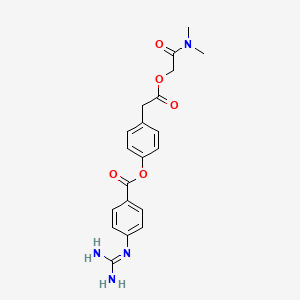

1. Camostat Mesilate
2. Camostat Mesylate
3. Camostate
4. Camostate-mesilate
5. Foipan
6. Foy 305
7. Foy S 980
8. Foy-305
9. Foypan
10. N,n-dimethylcarbamoylmethyl-4-(4-guanidinobenzoyloxy)phenylacetate Methanesulfonate
11. P-guanidinobenzoic Acid, Ester With (p-hydroxyphenyl)acetic Acid, Ester With N,n-dimethylglycolamide
1. 59721-28-7
2. Camostat [inn]
3. Camostate
4. [4-[2-[2-(dimethylamino)-2-oxoethoxy]-2-oxoethyl]phenyl] 4-(diaminomethylideneamino)benzoate
5. 0fd207wkdu
6. 4-(2-(2-(dimethylamino)-2-oxoethoxy)-2-oxoethyl)phenyl 4-guanidinobenzoate
7. Camostat (inn)
8. Foipan
9. P-guanidinobenzoic Acid, Ester With (p-hydroxyphenyl)acetic Acid, Ester With N,n-dimethylglycolamide
10. Camostatum
11. Camostatum [inn-latin]
12. Chembl85164
13. Ccris 7219
14. Ncgc00167526-01
15. Unii-0fd207wkdu
16. Camostat-mesilate
17. Dimethylcarbamoylmethyl 4-(4-guqnidinobenzoyloxy)phenylacetat
18. Camostat [mi]
19. Camostat [who-dd]
20. Us9199927, Camostat
21. Schembl125269
22. Chembl590799
23. Gtpl6432
24. Dtxsid6044010
25. Chebi:135632
26. Bdbm193418
27. Bcp22042
28. Ex-a5738
29. Zinc3871842
30. Bdbm50031706
31. Bdbm50424712
32. Bcp9000475
33. Db13729
34. Ncgc00167526-03
35. Db-053447
36. Ft-0630715
37. D07606
38. 721c287
39. Q5026909
40. 2-(dimethylamino)-2-oxoethyl4-(4-guanidinobenzoyloxy)phenylacetate
41. 4-(2-(2-(dimethylamino)-2-oxoethoxy)-2-oxoethyl)phenyl4-guanidinobenzoate
42. N,n-dimethyl Carbamoylmethyl-p-(p-guanidinobenzoyloxy) Phenyl-acetate
43. [4-[2-(2-dimethylamino-2-oxoethoxy)-2-oxoethyl]phenyl] 4-(diaminomethylideneamino)benzoate
44. 4-{2-[(dimethylcarbamoyl)methoxy]-2-oxoethyl}phenyl 4-carbamimidamidobenzoate
45. 4-guanidino-benzoic Acid 4-dimethylcarbamoylmethoxycarbonylmethyl-phenyl Ester; Compound With Methanesulfonic Acid
46. Benzeneacetic Acid,4-((4-((aminoiminomethyl)amino)benzoyl)oxy)-,2-(dimethylamino)-2-oxoethyl Ester
| Molecular Weight | 398.4 g/mol |
|---|---|
| Molecular Formula | C20H22N4O5 |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 9 |
| Exact Mass | 398.15901982 g/mol |
| Monoisotopic Mass | 398.15901982 g/mol |
| Topological Polar Surface Area | 137 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 602 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Camostat mesylate is indicated in Japan to treat chronic pancreatitis and drug induced lung injury. It is also being investigated as a potential treatment for COVID-19.
Camostat mesylate is a protease inhibitor used to treat chronic pancreatitis. The duration of action is not long, as it is typically given in 3 divided doses daily. Patients should be counselled regarding the risk of anaphylaxis, thrombocytopenia, hepatic dysfunction, and hyperkalemia.
Protease Inhibitors
Compounds which inhibit or antagonize biosynthesis or actions of proteases (ENDOPEPTIDASES). (See all compounds classified as Protease Inhibitors.)
Trypsin Inhibitors
Serine proteinase inhibitors which inhibit trypsin. They may be endogenous or exogenous compounds. (See all compounds classified as Trypsin Inhibitors.)
B - Blood and blood forming organs
B02 - Antihemorrhagics
B02A - Antifibrinolytics
B02AB - Proteinase inhibitors
B02AB04 - Camostat
Absorption
A 200mg oral dose of camostat mesylate leads to the active metabolite reaching a Cmax of 87.1 29.5 ng/mL, with a Tmax of 40 min, and an AUC of 10,400 1,400 ng\*min/mL.
Route of Elimination
Camostat mesylate is 89.8-95.6% eliminated in the urine and 1.0-1.7% eliminated in the feces.
Volume of Distribution
The volume of distribution at steady state of camostat mesylate is 0.34-1.31L/kg.
Clearance
The clearance of camostat mesylate is 4.5-7.3mL/min/kg.
Camostat mesylate is hydrolyzed by carboxyesterate to the active 4-(4-guanidinobenzoyloxy) phenylacetate. The active metabolite is further hydrolyzed by arylesterase to 4-guanidinobenzoic acid.
The half life of camostat mesylate is 3.8-4.7h.
In rats, oral camostat mesylate may increase pancreatic secretions and hypertrophy by increasing cholecystokinin release. Administration in rats has also lead to lower levels of IL-1beta, IL-6, TNF-alpha, TGF-beta, and PSC. Similar activity is seem after administration in humans, leading to reduced pain and inflammation as well as improve the function of the pancrease in chronic pancreatitis. In the case of SARS-CoV-2, camostat mesylate inhibits the action of the serine protease TMPRSS2, preventing the priming of the viral spike protein for attachment to ACE2, and entry into the cell.
ABOUT THIS PAGE
18
PharmaCompass offers a list of Camostatum API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Camostatum manufacturer or Camostatum supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Camostatum manufacturer or Camostatum supplier.
PharmaCompass also assists you with knowing the Camostatum API Price utilized in the formulation of products. Camostatum API Price is not always fixed or binding as the Camostatum Price is obtained through a variety of data sources. The Camostatum Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Camostatum manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Camostatum, including repackagers and relabelers. The FDA regulates Camostatum manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Camostatum API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Camostatum supplier is an individual or a company that provides Camostatum active pharmaceutical ingredient (API) or Camostatum finished formulations upon request. The Camostatum suppliers may include Camostatum API manufacturers, exporters, distributors and traders.
click here to find a list of Camostatum suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Camostatum Drug Master File in Japan (Camostatum JDMF) empowers Camostatum API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Camostatum JDMF during the approval evaluation for pharmaceutical products. At the time of Camostatum JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Camostatum suppliers with JDMF on PharmaCompass.
Camostatum Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Camostatum GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Camostatum GMP manufacturer or Camostatum GMP API supplier for your needs.
A Camostatum CoA (Certificate of Analysis) is a formal document that attests to Camostatum's compliance with Camostatum specifications and serves as a tool for batch-level quality control.
Camostatum CoA mostly includes findings from lab analyses of a specific batch. For each Camostatum CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Camostatum may be tested according to a variety of international standards, such as European Pharmacopoeia (Camostatum EP), Camostatum JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Camostatum USP).
