
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
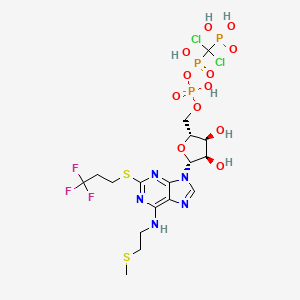

1. Ar C69931mx
2. Ar-c69931mx
3. Cangrelor Tetrasodium
4. Kengreal
5. N(6)-(2-methylthioethyl)-2-(3,3,3-trifluoropropylthio)-5'-adenylic Acid Monoanhydride With Dichloromethylenebis(phosphonic Acid)
1. 163706-06-7
2. Kengreal
3. Cangrelor Free Acid
4. Ar-c69931xx
5. Chembl334966
6. 6aq1y404u7
7. 163706-06-7 (free Acid)
8. (dichloro((((((2r,3s,4r,5r)-3,4-dihydroxy-5-(6-((2-(methylthio)ethyl)amino)-2-((3,3,3-trifluoropropyl)thio)-9h-purin-9-yl)tetrahydrofuran-2-yl)methoxy)(hydroxy)phosphoryl)oxy)(hydroxy)phosphoryl)methyl)phosphonic Acid
9. [dichloro-[[[(2r,3s,4r,5r)-3,4-dihydroxy-5-[6-(2-methylsulfanylethylamino)-2-(3,3,3-trifluoropropylsulfanyl)purin-9-yl]oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]methyl]phosphonic Acid
10. (dichloromethylene)diphosphonic N-(2-(methylsulfanyl)ethyl)-2-((3,3,3-trifluoropropyl)sulfanyl-5'-adenylic Monoanhydride
11. 5'-adenylicacid,n-[2-(methylthio)ethyl]-2-[(3,3,3-trifluoropropyl)thio]-,anhydridewithp,p'-(dichloromethylene)bis[phosphonicacid](1:1)
12. Cangrelor [usan:inn:ban]
13. Unii-6aq1y404u7
14. Arl69931
15. Cangrelor [inn]
16. Cangrelor [mi]
17. Cangrelor (usan/inn)
18. Cangrelor [usan]
19. Cangrelor [who-dd]
20. Gtpl1776
21. Schembl6113860
22. Cangrelor [orange Book]
23. Ammd00024
24. Arl 69931mx
25. Chebi:90841
26. Hsdb 8489
27. Dtxsid90167651
28. Ar-c-69931mx
29. Bdbm50118225
30. Mfcd09837758
31. Zinc85537017
32. Am85616
33. Db06441
34. Ncgc00480787-02
35. 5'-o-[({[dichloro(phosphono)methyl](hydroxy)phosphoryl}oxy)(hydroxy)phosphoryl]-n-[2-(methylsulfanyl)ethyl]-2-[(3,3,3-trifluoropropyl)sulfanyl]adenosine
36. Ac-28809
37. Hy-19638
38. Cs-0016143
39. D03359
40. Q3655338
41. 5'-adenylic Acid, N-(2-(methylthio)ethyl)-2-((3,3,3-trifluoropropyl)thio)-, Monoanhydride With (dichloromethylene)bis(phosphonic Acid)
42. 5'-adenylicacid, N-(2-(methylthio)ethyl)-2-((3,3,3-trifluoropropyl)thio)-, Monoanhydride With (dichloromethylene)bis(phosphonic Acid)
| Molecular Weight | 776.4 g/mol |
|---|---|
| Molecular Formula | C17H25Cl2F3N5O12P3S2 |
| XLogP3 | -1 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 21 |
| Rotatable Bond Count | 15 |
| Exact Mass | 774.9483145 g/mol |
| Monoisotopic Mass | 774.9483145 g/mol |
| Topological Polar Surface Area | 307 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 1140 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Platelet Aggregation Inhibitors; Purinergic P2Y Receptor Antagonists
National Library of Medicine's Medical Subject Headings. Cangrelor. Online file (MeSH, 2018). Available from, as of November 8, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Cangrelor is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of November 8, 2018: https://clinicaltrials.gov/
Kengreal is indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor. /Included in US product label/
NIH; DailyMed. Current Medication Information for Kengreal (Cangrelor Injection, Powder, Lyophilized, For Solution) (Updated: August 10, 2016). Available from, as of November 13, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=88b434fa-8891-4fd5-9d86-7ea64667c08f
The most common adverse effect of cangrelor reported in clinical trials was bleeding. Cases of transient dyspnea were also reported during clinical trials.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 1661
Hypersensitivity reactions (e.g., anaphylaxis, bronchospasm, angioedema, stridor) have been reported with cangrelor therapy.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 1660
Like other antiplatelet agents, cangrelor increases the risk of bleeding, which may be serious. In the CHAMPION PHOENIX trial, bleeding events of all severities were somewhat more common with cangrelor than with clopidogrel. In clinical trials, bleeding events in patients receiving cangrelor were mild, generally consisting of hematoma, ecchymosis, and oozing at the puncture site. In the CHAMPION PHOENIX trial, the rate of severe bleeding (per the Global Use of Strategies to Open Occluded Coronary Arteries [GUSTO] criteria) was not substantially increased by cangrelor, although the rate of major bleeding according to more sensitive criteria (Acute Catheterization and Urgent Intervention Triage Strategy [ACUITY]) was substantially higher with cangrelor than with clopidogrel (4.3 versus 2.5%). The increase in major bleeding with the ACUITY criteria was attributable to a greater incidence of hematoma at the site of vascular access in patients receiving cangrelor.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 1660
Cangrelor should not be used in patients with substantial active bleeding. The antiplatelet effects of cangrelor are negligible 1 hour after discontinuance of the infusion.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 1660
For more Drug Warnings (Complete) data for Cangrelor (11 total), please visit the HSDB record page.
For use as an adjunct to percutaneous coronary intervention (PCI) for reducing the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients in who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
FDA Label
Kengrexal, co-administered with acetylsalicylic acid (ASA), is indicated for the reduction of thrombotic cardiovascular events in adult patients with coronary artery disease undergoing percutaneous coronary intervention (PCI) who have not received an oral P2Y12 inhibitor prior to the PCI procedure and in whom oral therapy with P2Y12 inhibitors is not feasible or desirable.
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Purinergic P2Y Receptor Antagonists
Compounds that bind to and block the stimulation of PURINERGIC P2Y RECEPTORS. Included under this heading are antagonists for specific P2Y receptor subtypes. (See all compounds classified as Purinergic P2Y Receptor Antagonists.)
B01
B01AC25
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AC - Platelet aggregation inhibitors excl. heparin
B01AC25 - Cangrelor
Route of Elimination
Following IV administration of [3H] cangrelor, 58% of radioactivity was recovered in urine. The remaining 35% of radioactivity was in feces, presumably following biliary excretion.
Volume of Distribution
In a study in healthy volunteers administration at a dose of 30 mcg/kg bolus plus 4 mcg/kg/min showed a volume of distribution of 3.9 L.
Clearance
The mean clearance is about 43.2 L/h.
/MILK/ It is not known whether Kengreal is excreted in human milk.
NIH; DailyMed. Current Medication Information for Kengreal (Cangrelor Injection, Powder, Lyophilized, For Solution) (Updated: August 10, 2016). Available from, as of November 13, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=88b434fa-8891-4fd5-9d86-7ea64667c08f
Following IV administration of 3(H) Kengreal 58% of radioactivity was recovered in urine. The remaining 35% of radioactivity was in feces, presumably following biliary excretion. The average elimination half-life of Kengreal is about 3-6 minutes.
NIH; DailyMed. Current Medication Information for Kengreal (Cangrelor Injection, Powder, Lyophilized, For Solution) (Updated: August 10, 2016). Available from, as of November 13, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=88b434fa-8891-4fd5-9d86-7ea64667c08f
In a study in healthy volunteers, Kengreal administration at a dose of 30 ug/kg bolus plus 4 mcg/kg/min showed a volume of distribution of 3.9 L. Plasma protein binding of Kengreal is about 97-98%.
NIH; DailyMed. Current Medication Information for Kengreal (Cangrelor Injection, Powder, Lyophilized, For Solution) (Updated: August 10, 2016). Available from, as of November 13, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=88b434fa-8891-4fd5-9d86-7ea64667c08f
Cangrelor is deactivated rapidly in the circulation by dephosphorylation to its primary metabolite, a nucleoside, which has negligible anti-platelet activity. Cangrelor's metabolism is independent of hepatic function and it does not interfere with other drugs metabolized by hepatic enzymes.
Kengreal is deactivated rapidly in the circulation by dephosphorylation to its primary metabolite, a nucleoside, which has negligible anti-platelet activity. Kengreal's metabolism is independent of hepatic function and it does not interfere with other drugs metabolized by hepatic enzymes.
NIH; DailyMed. Current Medication Information for Kengreal (Cangrelor Injection, Powder, Lyophilized, For Solution) (Updated: August 10, 2016). Available from, as of November 13, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=88b434fa-8891-4fd5-9d86-7ea64667c08f
The average elimination half-life of cangrelor is about 3-6 minutes.
Following IV administration of 3(H) Kengreal, ... elimination half-life of Kengreal is about 3-6 minutes.
NIH; DailyMed. Current Medication Information for Kengreal (Cangrelor Injection, Powder, Lyophilized, For Solution) (Updated: August 10, 2016). Available from, as of November 13, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=88b434fa-8891-4fd5-9d86-7ea64667c08f
Cangrelor is a selective, reversible, P2Y12 platelet receptor antagonist which inhibits ADP platelet aggregation. ADP is typically released by damaged blood vessels, red blood cells, and/or platelets due to agonists stimulating platelet activity. ADP binds to P2Y12 to stimulate and complete platelet aggregation by inhibiting adenylyl cyclase by a Gi protein, thus potentiating dense granule secretion and increasing coagulation activity. Cangrelor acts on the same target as oral irreversible inhibitors clopidogrel and ticlopidine and has a similar mechanism of action, but is reversible and provides a fast onset and offset of action.
Cangrelor is a direct P2Y12 platelet receptor inhibitor that blocks ADP-induced platelet activation and aggregation. Cangrelor binds selectively and reversibly to the P2Y12 receptor to prevent further signaling and platelet activation.
NIH; DailyMed. Current Medication Information for Kengreal (Cangrelor Injection, Powder, Lyophilized, For Solution) (Updated: August 10, 2016). Available from, as of November 13, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=88b434fa-8891-4fd5-9d86-7ea64667c08f

517.0k
<10
0.3
1,60,259.8
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|---|---|---|
| INDIA | 0.30 | 4,51,669.3 | <10 |
| UNITED STATES | 0.01 | 24,75,900.0 | <10 |
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
18
PharmaCompass offers a list of Cangrelor API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Cangrelor manufacturer or Cangrelor supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Cangrelor manufacturer or Cangrelor supplier.
PharmaCompass also assists you with knowing the Cangrelor API Price utilized in the formulation of products. Cangrelor API Price is not always fixed or binding as the Cangrelor Price is obtained through a variety of data sources. The Cangrelor Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Cangrelor manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Cangrelor, including repackagers and relabelers. The FDA regulates Cangrelor manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Cangrelor API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Cangrelor manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Cangrelor supplier is an individual or a company that provides Cangrelor active pharmaceutical ingredient (API) or Cangrelor finished formulations upon request. The Cangrelor suppliers may include Cangrelor API manufacturers, exporters, distributors and traders.
click here to find a list of Cangrelor suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Cangrelor DMF (Drug Master File) is a document detailing the whole manufacturing process of Cangrelor active pharmaceutical ingredient (API) in detail. Different forms of Cangrelor DMFs exist exist since differing nations have different regulations, such as Cangrelor USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Cangrelor DMF submitted to regulatory agencies in the US is known as a USDMF. Cangrelor USDMF includes data on Cangrelor's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Cangrelor USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Cangrelor suppliers with USDMF on PharmaCompass.
A Cangrelor written confirmation (Cangrelor WC) is an official document issued by a regulatory agency to a Cangrelor manufacturer, verifying that the manufacturing facility of a Cangrelor active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Cangrelor APIs or Cangrelor finished pharmaceutical products to another nation, regulatory agencies frequently require a Cangrelor WC (written confirmation) as part of the regulatory process.
click here to find a list of Cangrelor suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Cangrelor as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Cangrelor API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Cangrelor as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Cangrelor and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Cangrelor NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Cangrelor suppliers with NDC on PharmaCompass.
Cangrelor Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Cangrelor GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Cangrelor GMP manufacturer or Cangrelor GMP API supplier for your needs.
A Cangrelor CoA (Certificate of Analysis) is a formal document that attests to Cangrelor's compliance with Cangrelor specifications and serves as a tool for batch-level quality control.
Cangrelor CoA mostly includes findings from lab analyses of a specific batch. For each Cangrelor CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Cangrelor may be tested according to a variety of international standards, such as European Pharmacopoeia (Cangrelor EP), Cangrelor JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Cangrelor USP).
