


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
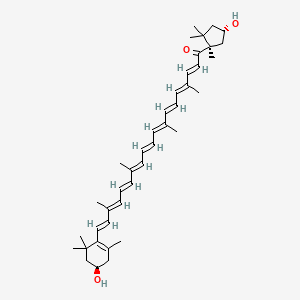

1. Capsanthin, (3r,3's,5'r,13-cis)-isomer
1. 465-42-9
2. Paprika Extract
3. All-trans-capsanthin
4. Capsanthin/capsorubin
5. (3r,3's,5'r)-3,3'-dihydroxy-beta,kappa-caroten-6'-one
6. Chebi:3375
7. 420ny1j57n
8. (2e,4e,6e,8e,10e,12e,14e,16e,18e)-19-[(4r)-4-hydroxy-2,6,6-trimethylcyclohexen-1-yl]-1-[(1r,4s)-4-hydroxy-1,2,2-trimethylcyclopentyl]-4,8,13,17-tetramethylnonadeca-2,4,6,8,10,12,14,16,18-nonaen-1-one
9. (2e,4e,6e,8e,10e,12e,14e,16e,18e)-1-((1r,4s)-4-hydroxy-1,2,2-trimethylcyclopentyl)-19-((r)-4-hydroxy-2,6,6-trimethylcyclohex-1-en-1-yl)-4,8,13,17-tetramethylnonadeca-2,4,6,8,10,12,14,16,18-nonaen-1-one
10. 3,3'-dihydroxy-beta,kappa-caroten-6'one
11. All Trans Capsanthin
12. Einecs 207-364-1
13. Unii-420ny1j57n
14. Hsdb 7977
15. Capsanthin [mi]
16. Beta,kappa-caroten-6'one, 3,3'dihydroxy-
17. Upcmld-dp025
18. Schembl117691
19. Spectrum1505276
20. Chembl1519371
21. Upcmld-dp025:001
22. Dtxsid10905012
23. Capsanthin, >=90.0% (hplc)
24. Zinc8221201
25. Capsanthin/capsorubin [inci]
26. Lmpr01070265
27. Mfcd31726206
28. Ccg-214238
29. Sdccgmls-0066894.p001
30. Ncgc00161603-01
31. Hy-125711
32. Cs-0093389
33. C08584
34. A827066
35. Q425431
36. Sr-05000002758
37. Sr-05000002758-1
38. 3,3'-dihydroxy-.beta.,.kappa.-caroten-6'on
39. Capsanthin (=paprika Extract)(vegetable Oil Solution)
40. .beta.,.kappa.-caroten-6'one, 3,3'dihydroxy-
41. (3r,3's,5'r)-3,3'-dihydroxy-.beta.,.kappa.-caroten-6'-one
42. (2e,4e,6e,8e,10e,12e,14e,16e,18e)-19-(4-hydroxy-2,6,6-trimethylcyclohexen-1-yl)-1-(4-hydroxy-1,2,2-trimethylcyclopentyl)-4,8,13,17-tetramethylnonadeca-2,4,6,8,10,12,14,16,18-nonaen-1-one
43. (2e,4e,6e,8e,10e,12e,14e,16e,18e)-19-[(4r)-4-hydroxy-2,6,6-trimethyl-1-cyclohexenyl]-1-[(1r,4s)-4-hydroxy-1,2,2-trimethylcyclopentyl]-4,8,13,17-tetramethyl-1-nonadeca-2,4,6,8,10,12,14,16,18-nonaenone
44. (2e,4e,6e,8e,10e,12e,14e,16e,18e)-4,8,13,17-tetramethyl-19-[(4r)-2,6,6-trimethyl-4-oxidanyl-cyclohexen-1-yl]-1-[(1r,4s)-1,2,2-trimethyl-4-oxidanyl-cyclopentyl]nonadeca-2,4,6,8,10,12,14,16,18-nonaen-1-one
45. 2,4,6,8,10,12,14,16,18-nonadecanonaen-1-one, 19-(4-hydroxy-2,6,6-trimethyl-1-cyclohexen-1-yl)-1-(4-hydroxy-1,2,2-trimethylcyclopentyl)-4,8,13,17-tetramethyl-, (all-e)-
46. 2,4,6,8,10,12,14,16,18-nonadecanonaen-1-one, 19-(4-hydroxy-2,6,6-trimethyl-1-cyclohexen-1-yl)-1-(5-hydroxy-1,2,2-trimethylcyclopentyl)-4,8,13,17-tetramethyl-
| Molecular Weight | 584.9 g/mol |
|---|---|
| Molecular Formula | C40H56O3 |
| XLogP3 | 10.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 11 |
| Exact Mass | 584.42294564 g/mol |
| Monoisotopic Mass | 584.42294564 g/mol |
| Topological Polar Surface Area | 57.5 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 1310 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 9 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
The pharmacokinetics of dietary capsanthin were determined in four male volunteers with plasma essentially free of capsanthin at the beginning of the study. They received paprika juice for 1 week, equivalent to three doses of 5.4 umol capsanthin/day, for a total of 16.2 umol/day. The level of capsanthin in plasma reached a plateau (0.10-0.12 umol/L) between day 2 and day 7, and capsanthin was not detectable in plasma by day 16. Capsanthin was distributed in the plasma lipoproteins after 1 week as follows: very low density lipoprotein, 13 +/- 3%; low-density lipoprotein, 44 +/- 3%; and high-density lipoprotein, 43 +/- 3%.
WHO/FAO; Joint Expert Committee on Food Additives (JECFA): Food Additive Series 60: Safety evaluation of certain food additives. Available from, As of June 22, 2011: https://www.inchem.org/pages/jecfa.html
In a /study/ involving the single ingestion of paprika juice (equivalent to 34.2 umol capsanthin) by the same men, the plasma concentration of capsanthin ranged from 0.10 to 0.29 umol/L at 8 hr after ingestion. In contrast, the elevation of the plasma concentration of an acyclic hydrocarbon carotenoid, lycopene, by a single ingestion of tomato soup (equivalent to 186.3 mmol lycopene) in the same subjects was minimal (0.02-0.06 mmol/L). The areas under the plasma concentration-time curves for capsanthin between 0 and 74 hr and for lycopene between 0 and 72 hr were 4.68 +/- 1.22 and 0.81 +/- 0.17 (umol.hr)/L, respectively. The half-lives were calculated to be 20.1 +/- 1.3 hr for capsanthin and 222 +/- 15 hr for lycopene. It was concluded that the clearance of capsanthin is much faster than that of lycopene, although capsanthin is transported into plasma lipoproteins in larger amounts.
WHO/FAO; Joint Expert Committee on Food Additives (JECFA): Food Additive Series 60: Safety evaluation of certain food additives. Available from, As of June 22, 2011: https://www.inchem.org/pages/jecfa.html
The bioavailability of carotenoids from a paprika oleoresin (zeaxanthin, beta- cryptoxanthin, beta-carotene, capsanthin, capsorubin) was assessed in humans. After overnight fasting, nine volunteers ingested a single dose of a paprika oleoresin containing 6.4 mg zeaxanthin, 4.2 mg beta-cryptoxanthin, 6.2 mg beta-carotene, 35.0 mg capsanthin and 2.0 mg capsorubin. At different time points, the carotenoid pattern in the chylomicron fraction of whole blood was analyzed to evaluate carotenoid absorption. From the major carotenoids present in the paprika oleoresin, only zeaxanthin, beta-cryptoxanthin and beta-carotene were detectable in measurable amounts. Although the xanthophylls in paprika oleoresin were mainly present as mono- or diesters, only free zeaxanthin and beta-cryptoxanthin were found. The bioavailability of the pepper-specific carotenoids capsanthin and capsorubin from paprika oleoresin was found to be very low.
WHO/FAO; Joint Expert Committee on Food Additives (JECFA): Food Additive Series 60: Safety evaluation of certain food additives. Available from, As of June 22, 2011: https://www.inchem.org/pages/jecfa.html
/A/ study in which rats were gavaged with a mixture of capsaicinoids /was reported/. These substances were extensively metabolized by a variety of metabolic pathways, including 1) hydrolysis of the acid-amide bond and deamination to form vanillylamine, 2) hydroxylation of the vanillyl ring, 3) oxidation of the hydroxyl group in the ring and 4) oxidation of the terminal carbon in the sidechain. /Capsaicinoids/
WHO/FAO; Joint Expert Committee on Food Additives (JECFA): Food Additive Series 60: Safety evaluation of certain food additives. Available from, As of June 22, 2011: https://www.inchem.org/pages/jecfa.html
Later steps of carotenoid biosynthesis catalyzed by cyclase enzymes involve the formation of alpha, beta, and kappa-rings. Examination of the primary structure of lycopene beta-cyclase revealed 55% identity with that of antheraxanthin kappa-cyclase. Recombinant lycopene beta-cyclase afforded only beta-carotene, while recombinant antheraxanthin kappa-cyclase catalyzed the formation of beta-carotene from lycopene as well as the conversion of antheraxanthin into the kappa-carotenoid capsanthin. Since the formation of beta- and kappa-rings involves a transient carotenoid carbocation, this suggests that both cyclases initiate and/or neutralize the incipient carbocation by similar mechanisms. Several amine derivatives protonated at physiological pH were used to examine the molecular basis of this phenomenon. The beta-and kappa-cyclases displayed similar inhibition patterns. Affinity or photoaffinity labeling using p-dimethylamino-benzenediazonium fluoroborate, N,N-dimethyl-2-phenylaziridinium, and nicotine irreversibly inactivated both cyclase enzymes. Photoaffinity labeling using [H(3)]nicotine followed by radiosequence analysis and site-directed mutagenesis revealed the existence of two cyclase domains characterized by the presence of reactive aromatic and carboxylic amino acid residues. /Investigators/ propose that these residues represent the "negative point charges" involved in the coordination of the incipient carotenoid carbocations.
PMID:9328284 Bouvier F et al; 346 (1): 53-64 (1997)
... In a /study/ involving the single ingestion of paprika juice (equivalent to 34.2 umol capsanthin) The half-lives /after a single ingestion of paprika juice (equivalent to 34.2 umol capsanthin)/ were calculated to be 20.1 +/- 1.3 hr for capsanthin and 222 +/- 15 hr for lycopene. ...
WHO/FAO; Joint Expert Committee on Food Additives (JECFA): Food Additive Series 60: Safety evaluation of certain food additives. Available from, As of June 22, 2011: https://www.inchem.org/pages/jecfa.html


