
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
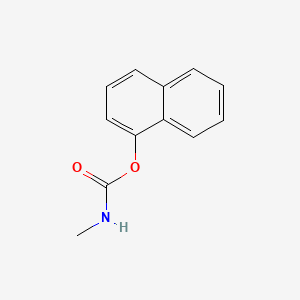

1. 1-naphthyl-n-methylcarbamate
2. Antigale
3. Carbaril
4. Carbaryl, Manganese (2+) Salt
5. Carbaryl, Nickel (2+) Salt
6. Carbyl
7. Carylderm
8. Concentrat Vo 18
9. Dog Net Insecticide Poudre
10. Dog-net Insecticide Poudre
11. Dognet Insecticide Poudre
12. Fido's Free Itch
13. Fido's Free-itch
14. Fido's Freeitch
15. G Wizz
16. G-wizz
17. Gwizz
18. Insecticide Moureau, Poudre
19. Insecticide Vetoquinol, Poudre
20. Joseph Lyddy
21. Lyddy, Joseph
22. Moureau, Poudre Insecticide
23. Occoxil
24. Poudre Insecticide Moureau
25. Poudre Insecticide Vetoquinol
26. Poutic
27. Spou
28. Sevin
29. Skatta Tick Flea Louse Powder
30. Tigal
31. Vetoquinol, Poudre Insecticide
32. Vo 18, Concentrat
1. 63-25-2
2. Carbaril
3. Sevin
4. 1-naphthyl N-methylcarbamate
5. Naphthalen-1-yl Methylcarbamate
6. 1-naphthyl Methylcarbamate
7. Arilat
8. Caprolin
9. Carbatox
10. Carbavur
11. Carylderm
12. Menaphtam
13. 1-naphthalenol, Methylcarbamate
14. Carbomate
15. Carpolin
16. Dicarbam
17. Karbaspray
18. Karbatox
19. Karbosep
20. Tricarnam
21. Arylam
22. Atoxan
23. Denapon
24. Gamonil
25. Hexavin
26. Monsur
27. Murvin
28. Ravyon
29. Seffein
30. Septene
31. Sevimol
32. Vioxan
33. Panam
34. Pomex
35. Suleo
36. Dyna-carbyl
37. Crag Sevin
38. Germain's
39. N-methyl-1-naphthyl Carbamate
40. Carbamine
41. Carbatox-60
42. Karbatox 75
43. Oltitox
44. Mugan
45. Vetox
46. Bercema Nmc50
47. Compound 7744
48. Naphthalen-1-yl N-methylcarbamate
49. Prosevor 85
50. Carbaril [inn]
51. Dicarbament 23,969
52. 1-naphthol N-methylcarbamate
53. Cekubaryl
54. Devicarb
55. Karbaryl
56. Olititox
57. Sevin 4
58. Crunch
59. Tercyl
60. Rylam
61. Savit
62. Toxan
63. 1-naphthalenyl Methylcarbamate
64. Bug Master
65. 1-naphthyl-n-methylcarbamate
66. Carbatox-75
67. N-methyl-alpha-naphthylurethan
68. Noflo 5 Vet
69. Karbaryl [polish]
70. Union Carbide 7,744
71. Karbatox Zawiesinowy
72. Oms-29
73. Alpha-naphthyl N-methylcarbamate
74. Carbarilo
75. Carbarilum
76. Caswell No. 160
77. Ent-23969
78. Experimental Insecticide 7744
79. Nmc 50
80. Carbatox 75
81. Methylcarbamate 1-naphthol
82. N-methyl-1-naftyl-carbamaat
83. N-metil-1-naftil-carbammato
84. Methylcarbamate 1-naphthalenol
85. N-methyl-1-naphthyl-carbamat
86. Methylcarbamic Acid, 1-naphthyl Ester
87. Sewin
88. N-methylcarbamate De 1-naphtyle
89. Uc 7744
90. 1-naphthalenol, 1-(n-methylcarbamate)
91. Nsc-27311
92. Carbamic Acid, Methyl-, 1-naphthyl Ester
93. Chembl46917
94. Chebi:3390
95. .alpha.-naftyl-n-methylkarbamat
96. .alpha.-naphthyl Methylcarbamate
97. N-methyl-.alpha.-naphthylurethan
98. R890c8j3n1
99. .alpha.-naphthyl N-methylcarbamate
100. N-methyl-.alpha.-naphthylcarbamate
101. .alpha.-naphthalenyl Methylcarbamate
102. Ncgc00090680-01
103. Derbac
104. Dsstox_cid_247
105. Carbaryl (sevin)
106. Dsstox_rid_75461
107. Dsstox_gsid_20247
108. Nac (insecticide)
109. Carbaril [italian]
110. Clinicide
111. Thinsec
112. Adios
113. Carbarilum [inn-latin]
114. Carbarilo [inn-spanish]
115. Carbaryl Solution
116. Latka 7744 [czech]
117. Nac (van)
118. Cas-63-25-2
119. Carbaryl [ansi:bsi:iso]
120. Alpha-naphthyl Methylcarbamate
121. Ccris 850
122. 1-naphthalenol Methylcarbamate
123. (14co)-carbaryl
124. Hsdb 952
125. Oms 29
126. 1433961-56-8
127. N-methyl-alpha-naphthylcarbamate
128. Alpha-naphthalenyl Methylcarbamate
129. O-(1-naphthyl)-n-methylcarbamat
130. Latka 7744
131. Einecs 200-555-0
132. Ent 23969
133. N-methyl Naphthylcarbamate
134. Naphthyl N-methylcarbamate
135. Nsc 27311
136. N-methyl-1-naftyl-carbamaat [dutch]
137. Alpha-naftyl-n-methylkarbamat [czech]
138. Ent 23,969
139. Epa Pesticide Chemical Code 056801
140. 1-naphthyl-n-methyl-karbamat [german]
141. N-methyl-1-naphthyl-carbamat [german]
142. N-metil-1-naftil-carbammato [italian]
143. Brn 1875862
144. Naphthalenol, Methylcarbamate
145. Alpha-naftyl-n-methylkarbamat
146. 1-naphthyl-n-methyl-karbamat
147. Laivin
148. N-methylcarbamate De 1-naphtyle [french]
149. Unii-r890c8j3n1
150. Ai3-23969
151. 1-naphthyl Methylcarbamate-14c
152. Sevin Sl
153. 1-naftylester Kyseliny Methylkarbaminove [czech]
154. Carbaryl (ban)
155. Vetox 85
156. 3197-92-0
157. 1-naftylester Kyseliny Methylkarbaminove
158. Union Carbide 7744
159. Carbaryl [hsdb]
160. Carbaryl [iarc]
161. Carbaryl [iso]
162. Carbaryl [mi]
163. Maybridge3_000390
164. Carbaryl [mart.]
165. Carbaril [who-dd]
166. Carbamic-14c Acid, Methyl-, 1-naphthyl Ester
167. Cid_6129
168. Wln: L66j Bovm1
169. Schembl26737
170. 27636-33-5
171. Carbamic Acid, Methyl-, Naphthalenyl Ester
172. Mls000851157
173. Bidd:er0592
174. Methylcarbamate, 1-naphthalenol
175. Zinc1090
176. Alpha-naphthyl-n-methylcarbamate
177. Dtxsid9020247
178. Cvxbeemkqhexen-uhfffaoysa-
179. Hms1432b16
180. Hms2809n24
181. Bcp18824
182. Hy-b1315
183. Nsc27311
184. 1-naphthyl-n-methylcarbamate, 97%
185. Tox21_110994
186. Tox21_201836
187. Tox21_300854
188. Bdbm50128572
189. Ccg-41182
190. Mfcd00021467
191. S5424
192. Stl371215
193. Carbamic Acid, N-methyl-1-naphthyl-
194. Carbaryl 10 Microg/ml In Cyclohexane
195. Akos001115604
196. Tox21_110994_1
197. Carbaryl 100 Microg/ml In Cyclohexane
198. Gs-3217
199. 1-naphthalenyl N-methylcarbamate
200. Idi1_011777
201. Flea And Tick Powder [veterinary] (tn)
202. Ncgc00090680-02
203. Ncgc00090680-03
204. Ncgc00090680-04
205. Ncgc00090680-05
206. Ncgc00090680-06
207. Ncgc00254757-01
208. Ncgc00259385-01
209. Carbamic Acid, N-methyl,1-naphthyl Ester
210. Smr000457400
211. Db-054440
212. C3742
213. Carbaryl, Pestanal(r), Analytical Standard
214. Cs-0013076
215. Ft-0608122
216. Ft-0664245
217. En300-06105
218. C07491
219. D07613
220. H11927
221. Ab00648589_06
222. 021c467
223. Q415090
224. Sr-01000631277
225. 1-naphthyl N-methylcarbamateacid O,o-diethyl Ester
226. Sr-01000631277-1
227. Sr-01000631277-3
228. Z90123586
229. Carbaryl Solution, 100 Mug/ml In Cyclohexane, Pestanal(r), Analytical Standard
| Molecular Weight | 201.22 g/mol |
|---|---|
| Molecular Formula | C12H11NO2 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 201.078978594 g/mol |
| Monoisotopic Mass | 201.078978594 g/mol |
| Topological Polar Surface Area | 38.3 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 230 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Formulations of carbaryl have been used successfully to control human lice.
Hayes, W.J., Jr., E.R. Laws, Jr., (eds.). Handbook of Pesticide Toxicology. Volume 3. Classes of Pesticides. New York, NY: Academic Press, Inc., 1991., p. 1152
/VETERINARY ANIMALS/ To control fleas, lice, ticks, & mites on animals, poultry, & premises, incl sarcoptic mange on buffaloes; lice, ticks, & mange mites on cattle; fleas & resistant fleas on dogs; & fowl mites, lice, & fleas on poultry.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 79
Medication (Vet): Ectoparasiticide
Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 290
Insecticides
Pesticides designed to control insects that are harmful to man. The insects may be directly harmful, as those acting as disease vectors, or indirectly harmful, as destroyers of crops, food products, or textile fabrics. (See all compounds classified as Insecticides.)
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)
An in vitro dermal penetration study was conducted using both rat and human skin . This study showed that rat skin was 2.8 times more permeable to carbaryl than human skin.
USEPA/Office of Prevention, Pesticides and Toxic Substances; Reregistration Eligibility Decision for Carbaryl. p 8 .EPA-738RO7-018 (September 2007) Available from, as of November 4, 2008: https://www.epa.gov/oppsrrd1/REDs/carbaryl_red.pdf
Excretion--retention of ... carbaryl labeled in 3 different positions ... studied 48 hr after ip admin to rats. 65% of (14)C of carbonyl-(14)C-carbaryl was excreted in urine, 25% in expired air, 2% in feces, & 10% was retained ... highest levels of (14)C were present in liver, kidneys, heart, & corpuscles (erythrocytes & leukocytes). 58% of (14)C of n-methyl-(14)C-carbaryl was excreted in urine, 12% in expired air, 4% in feces, & 13% was retained ... (14)C was maximal in liver, kidneys, heart, lungs, & spleen, organs with high blood flow. 77% of (14)C of naphthyl-(14)C-carbaryl was excreted in urine, 9% in feces, & 7% was retained ... levels of (14)C in tissue were highest in kidneys, spleen, bone, & fat ... about 50% of (14)C had been excreted in 4 hr ... .
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 81
Of oral dose of (1-naphthyl-1-(14)C)-N-methylcarbamate given to rats, 53% & 82% were absorbed after 20 min & 1 hr, respectively. Carbaryl is absorbed very rapidly from lung, 2.5 times faster than from small intestine.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V12 43 (1976)
(14)C-carbaryl labeled in n-methyl group has been found in fetuses of pregnant rats, & mice. ... Autoradiographic study of (14)C-methyl-carbaryl in pregnant rat has shown that radiolabel was localized in eye, liver, & brain of fetus.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 5: A Review of the Literature Published during 1976 and 1977. London: The Chemical Society, 1979., p. 458
For more Absorption, Distribution and Excretion (Complete) data for CARBARYL (9 total), please visit the HSDB record page.
Sevin, labeled with (14)C, is metabolized in insects, in rat, and by microsomal prepn of mouse, rat & rabbit liver, by aromatic hydroxylation, n-methyl hydroxylation, hydrolysis of carbamyl grouping, & by conjugation.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 205
Of the metabolites identified in livestock commodities, five are considered significant ... : carbaryl; 5,6-dihydro-5,6-dihydroxy carbaryl; and 5-methoxy-6-hydroxy carbaryl and all residues which can be hydrolyzed to carbaryl, 5,6-dihydro-5,6-dihydroxy carbaryl, or 5-methoxy-6-hydroxy carbaryl under acidic conditions. The /EPA/ included these compounds in the dietary risk assessment for carbaryl, and in the reassessment of carbaryl tolerances for livestock commodities only.
USEPA/Office of Pesticides Programs; Interim Reregistration Eligibility Decision for Carbaryl. p.12 (October 22, 2004) Available at https://www.epa.gov/oppsrrd1/REDs/carbaryl_ired.pdf on November 4, 2008
Single oral dose of carbaryl was admin to rats. After extraction of ... urine, column & TLC chromotography, tentative identification was made for 1,5-naphthalenediol with small amt of carbaryl, 5-hydroxycarbaryl, & trace of n-hydroxymethylcarbaryl was also present. A major metab ... identified as 5,6-dihydro-5,6-dihydroxycarbaryl, was found free (1.4% of the dose) & as the glucuronide (10.5% of the dose). Naphthyl glucuronide & sulfate were also observed.
Menzie, C. M. Metabolism of Pesticides, An Update. U.S. Department of the Interior, Fish, Wild-life Service, Special Scientific Report - Wildlife No. 184, Washington, DC: U.S. Government Printing Office, l974., p. 73
In urine of rats in addn to 1-naphthol, 1-naphthyl methylcarbamate N-glucuronide, 1-naphthyl methylimido-carbonate O-glucuronide, 4-(methylcarbamoyloxy)-1-naphthyl glucuronide, 1-naphthyl glucuronide, 1-naphthyl sulfate, 4-(methylcarbamoyloxy)-1-naphthyl sulfate, 3 unidentified cmpd & cmpd believed to be 1-naphthyl N-hydroxymethylcarbamate were observed. Similar results were observed with guinea pigs.
Menzie, C.M. Metabolism of Pesticides. U.S. Department of the Interior, Bureau of Sport Fisheries and Wildlife, Publication 127. Washington, DC: U.S. Government Printing Office, 1969., p. 72
For more Metabolism/Metabolites (Complete) data for CARBARYL (17 total), please visit the HSDB record page.
Carbaryl has known human metabolites that include 4-hydroxycarbaryl, 5-hydroxycarbaryl, and carbaryl methylol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
... The half-life of carbaryl was 6.4 min in the empty small intestines and 2.6 min in the lungs of rats.
Hayes, W.J., Jr., E.R. Laws, Jr., (eds.). Handbook of Pesticide Toxicology. Volume 3. Classes of Pesticides. New York, NY: Academic Press, Inc., 1991., p. 1146
ABOUT THIS PAGE
78
PharmaCompass offers a list of Carbaryl API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Carbaryl manufacturer or Carbaryl supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Carbaryl manufacturer or Carbaryl supplier.
PharmaCompass also assists you with knowing the Carbaryl API Price utilized in the formulation of products. Carbaryl API Price is not always fixed or binding as the Carbaryl Price is obtained through a variety of data sources. The Carbaryl Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Carbaryl manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Carbaryl, including repackagers and relabelers. The FDA regulates Carbaryl manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Carbaryl API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Carbaryl supplier is an individual or a company that provides Carbaryl active pharmaceutical ingredient (API) or Carbaryl finished formulations upon request. The Carbaryl suppliers may include Carbaryl API manufacturers, exporters, distributors and traders.
Carbaryl Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Carbaryl GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Carbaryl GMP manufacturer or Carbaryl GMP API supplier for your needs.
A Carbaryl CoA (Certificate of Analysis) is a formal document that attests to Carbaryl's compliance with Carbaryl specifications and serves as a tool for batch-level quality control.
Carbaryl CoA mostly includes findings from lab analyses of a specific batch. For each Carbaryl CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Carbaryl may be tested according to a variety of international standards, such as European Pharmacopoeia (Carbaryl EP), Carbaryl JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Carbaryl USP).
