

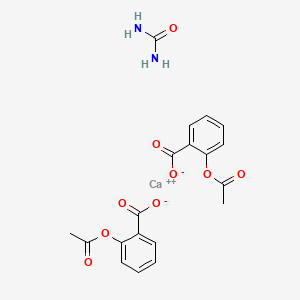

1. Calcium Acetylsalicylate Complex With Urea
2. Calcium Acetylsalicylic Carbamidate
3. Calcium Carbasalate
4. Calurin
5. Carbaspirin Calcium
6. Carbosalate Calcium
1. 5749-67-7
2. Carbaspirin Calcium
3. Alcacyl
4. Rheomin
5. Solupsan
6. Iromin
7. Omegin
8. Calcium Carbaspirin
9. Carbaspirin Calcium [usan]
10. Calcium Acetylsalicylate Complex With Urea
11. Carbasalate Calcium [inn]
12. N667f17jp1
13. Benzoic Acid, 2-(acetyloxy)-, Calcium Salt, Compd. With Urea (1:1)
14. Salicylic Acid Acetate Calcium Salt, Compound With Urea (1:1) Complex
15. Carbasalate Calcium (inn)
16. Carbaspirin Calcium (usan)
17. Carbasalate Calcique
18. Calpirinsan
19. Carbasalatcalcium
20. Carbaspirin Calium
21. Calcium;2-acetyloxybenzoate;urea
22. Carbasalato Calcico
23. Carbasalatum Calcicum
24. Unii-n667f17jp1
25. Carbasalate Calcique [inn-french]
26. Carbasalato Calcico [inn-spanish]
27. Carbasalatum Calcicum [inn-latin]
28. Carbasalate?calcium
29. Calcium; 2-acetyloxybenzoate; Urea
30. Einecs 227-273-0
31. Chembl3833325
32. Dtxsid90206099
33. Alcacyl; Rheomin; Solupsan; Omegin
34. Bcp12255
35. Carbasalate Calcium [mart.]
36. Carbasalate Calcium [who-dd]
37. Akos015895683
38. Akos025401461
39. Db13612
40. Calcium Acetylsalicylate Carbamide
41. Ac-18297
42. As-13259
43. Carbasalate Calcium [ep Monograph]
44. D03385
45. A831468
46. Q2203957
47. Calcium Acetylsalicylate Complex With Urea [mi]
| Molecular Weight | 458.4 g/mol |
|---|---|
| Molecular Formula | C19H18CaN2O9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 4 |
| Exact Mass | 458.0638210 g/mol |
| Monoisotopic Mass | 458.0638210 g/mol |
| Topological Polar Surface Area | 202 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 235 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AC - Platelet aggregation inhibitors excl. heparin
B01AC08 - Carbasalate calcium
N - Nervous system
N02 - Analgesics
N02B - Other analgesics and antipyretics
N02BA - Salicylic acid and derivatives
N02BA15 - Carbasalate calcium

LOOKING FOR A SUPPLIER?