
Synopsis
Synopsis
0
JDMF
0
KDMF
0
NDC API
0
FDA Orange Book

0
Canada

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Carbimazole Henning
2. Neo Tomizol
3. Neo-mercazole
4. Neo-thyreostat
5. Neomercazole
1. 22232-54-8
2. Athyromazole
3. Carbimazol
4. Neomercazole
5. Carbethoxymethimazole
6. Carbinazole
7. Neo-thyreostat
8. Basolest
9. Thyrostat
10. Carbimazolum
11. Tyrazol
12. Carbimazolum [inn-latin]
13. Ethyl 3-methyl-2-thioimidazoline-1-carboxylate
14. Carbimazol [inn-spanish, French]
15. Carbimazol Henning
16. 1-ethoxycarbonyl-3-methyl-2-thioxo-4-imidazoline
17. 1h-imidazole-1-carboxylic Acid, 2,3-dihydro-3-methyl-2-thioxo-, Ethyl Ester
18. Ethyl 3-methyl-2-thioxo-2,3-dihydro-1h-imidazole-1-carboxylate
19. Carbimazole (inn)
20. Neo-mercazole
21. Chebi:617099
22. Atirozidina
23. Carbotiroid
24. Mertiran
25. Cg1
26. 3-methyl-2-thioxo-4-imidazoline-1-carboxylic Acid Ethyl Ester
27. Nsc-758966
28. 4-imidazoline-1-carboxylic Acid, 3-methyl-2-thioxo-, Ethyl Ester
29. Carbimazol Spofa
30. Neo-tireol
31. Ethyl 3-methyl-2-sulfanylideneimidazole-1-carboxylate
32. Chembl508102
33. 8kq660g60g
34. Ethyl 3-methyl-2-thionoimidazoline-1-carboxylate
35. Ncgc00016760-01
36. Cas-22232-54-8
37. Carbimazole [inn]
38. Dsstox_cid_2736
39. Dsstox_rid_76708
40. Dsstox_gsid_22736
41. Carbimazole [inn:ban:dcf]
42. 1-ethoxycarbonyl-3-methyl-2-thioimidazol
43. Sr-01000872660
44. Carbimazol Henning (tn)
45. Einecs 244-854-4
46. Brn 0144339
47. Unii-8kq660g60g
48. Prestwick_788
49. 1h-imidazole-1-carboxylic Acid, 2,3-dihydro-3-methyl-2-thioxo, Ethyl Ester
50. Mfcd00027421
51. Fenobucarb Bassa Bpmc
52. Carbimazole [mi]
53. Prestwick0_000439
54. Prestwick1_000439
55. Prestwick2_000439
56. Prestwick3_000439
57. Spectrum2_001251
58. Spectrum3_001968
59. Epitope Id:116890
60. Carbimazole [mart.]
61. Schembl44211
62. Bspbio_000458
63. Bspbio_003568
64. Carbimazole [who-dd]
65. 4-24-00-00064 (beilstein Handbook Reference)
66. Mls004734638
67. Spectrum1505323
68. Spbio_001182
69. Spbio_002397
70. Bpbio1_000504
71. Zinc1091
72. Dtxsid9022736
73. Kbio3_002918
74. Carbimazole [ep Monograph]
75. Hms1569g20
76. Hms1922d12
77. Hms2096g20
78. Hms3652g04
79. Hms3713g20
80. Pharmakon1600-01505323
81. Bcp12935
82. Hy-b0558
83. Tox21_110596
84. Tox21_302320
85. Bdbm50275889
86. Ccg-39106
87. Ethyl 3-methyl-2-sulfanylidene-2,3-dihydro-1h-imidazole-1-carboxylate
88. Nsc758966
89. S4048
90. Akos001647527
91. Tox21_110596_1
92. Ac-8348
93. Am84794
94. Db00389
95. Nsc 758966
96. Ncgc00016760-02
97. Ncgc00016760-03
98. Ncgc00016760-04
99. Ncgc00016760-05
100. Ncgc00016760-08
101. Ncgc00095167-01
102. Ncgc00095167-02
103. Ncgc00177988-01
104. Ncgc00177988-02
105. Ncgc00255133-01
106. As-13267
107. Be164282
108. Smr001233183
109. Sbi-0207026.p001
110. Ft-0602919
111. Sw196926-3
112. A16427
113. C07615
114. C74138
115. D07616
116. 3-methyl-2-thioi-midazoline-1-carboxylate
117. A816030
118. Q414013
119. Sr-01000872660-1
120. Sr-01000872660-2
121. W-107493
122. Brd-k87156652-001-05-1
123. Brd-k87156652-001-06-9
124. Ethyl 3-methyl-2-thioxo-imidazole-1-carboxylate;carbimazole
125. Ethyl 3-methyl-2-thioxo-2,3-dihydro-1h-imidazole-1-carboxylate #
126. Carbimazole 3-methyl-2-thionoimidazoline-1-carboxylic Acid Ethyl Ester
127. Ethyl 3-methyl-2-thioxo-2,3-dihydro-1h-imidazole-1-carboxylate, Aldrichcpr
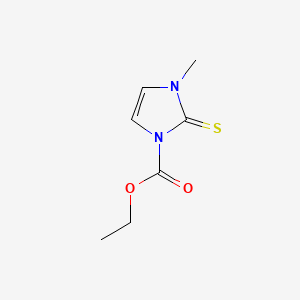
| Molecular Weight | 186.23 g/mol |
|---|---|
| Molecular Formula | C7H10N2O2S |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 186.04629874 g/mol |
| Monoisotopic Mass | 186.04629874 g/mol |
| Topological Polar Surface Area | 64.9 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 240 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of hyperthyroidism and thyrotoxicosis. It is also used to prepare patients for thyroidectomy.
Carbimazole is a carbethoxy derivative of methimazole. Its antithyroid action is due to its conversion to methimazole after absorption. It is used to treat hyperthyroidism and thyrotoxicosis.
Antithyroid Agents
Agents that are used to treat hyperthyroidism by reducing the excessive production of thyroid hormones. (See all compounds classified as Antithyroid Agents.)
H - Systemic hormonal preparations, excl. sex hormones and insulins
H03 - Thyroid therapy
H03B - Antithyroid preparations
H03BB - Sulfur-containing imidazole derivatives
H03BB01 - Carbimazole
Carbimazole is an aitithyroid agent that decreases the uptake and concentration of inorganic iodine by thyroid, it also reduces the formation of di-iodotyrosine and thyroxine. Once converted to its active form of methimazole, it prevents the thyroid peroxidase enzyme from coupling and iodinating the tyrosine residues on thyroglobulin, hence reducing the production of the thyroid hormones T3 and T4.
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
53
PharmaCompass offers a list of Carbimazole API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Carbimazole manufacturer or Carbimazole supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Carbimazole manufacturer or Carbimazole supplier.
PharmaCompass also assists you with knowing the Carbimazole API Price utilized in the formulation of products. Carbimazole API Price is not always fixed or binding as the Carbimazole Price is obtained through a variety of data sources. The Carbimazole Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Carbimazole manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Carbimazole, including repackagers and relabelers. The FDA regulates Carbimazole manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Carbimazole API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Carbimazole manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Carbimazole supplier is an individual or a company that provides Carbimazole active pharmaceutical ingredient (API) or Carbimazole finished formulations upon request. The Carbimazole suppliers may include Carbimazole API manufacturers, exporters, distributors and traders.
click here to find a list of Carbimazole suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Carbimazole DMF (Drug Master File) is a document detailing the whole manufacturing process of Carbimazole active pharmaceutical ingredient (API) in detail. Different forms of Carbimazole DMFs exist exist since differing nations have different regulations, such as Carbimazole USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Carbimazole DMF submitted to regulatory agencies in the US is known as a USDMF. Carbimazole USDMF includes data on Carbimazole's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Carbimazole USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Carbimazole suppliers with USDMF on PharmaCompass.
A Carbimazole CEP of the European Pharmacopoeia monograph is often referred to as a Carbimazole Certificate of Suitability (COS). The purpose of a Carbimazole CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Carbimazole EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Carbimazole to their clients by showing that a Carbimazole CEP has been issued for it. The manufacturer submits a Carbimazole CEP (COS) as part of the market authorization procedure, and it takes on the role of a Carbimazole CEP holder for the record. Additionally, the data presented in the Carbimazole CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Carbimazole DMF.
A Carbimazole CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Carbimazole CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Carbimazole suppliers with CEP (COS) on PharmaCompass.
A Carbimazole written confirmation (Carbimazole WC) is an official document issued by a regulatory agency to a Carbimazole manufacturer, verifying that the manufacturing facility of a Carbimazole active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Carbimazole APIs or Carbimazole finished pharmaceutical products to another nation, regulatory agencies frequently require a Carbimazole WC (written confirmation) as part of the regulatory process.
click here to find a list of Carbimazole suppliers with Written Confirmation (WC) on PharmaCompass.
Carbimazole Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Carbimazole GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Carbimazole GMP manufacturer or Carbimazole GMP API supplier for your needs.
A Carbimazole CoA (Certificate of Analysis) is a formal document that attests to Carbimazole's compliance with Carbimazole specifications and serves as a tool for batch-level quality control.
Carbimazole CoA mostly includes findings from lab analyses of a specific batch. For each Carbimazole CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Carbimazole may be tested according to a variety of international standards, such as European Pharmacopoeia (Carbimazole EP), Carbimazole JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Carbimazole USP).
