
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
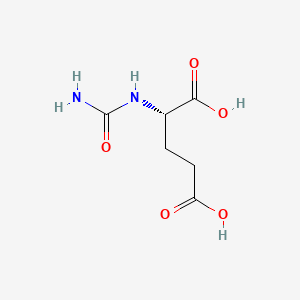

1. Carbamylglutamate
2. N-carbamoyl-l-glutamate
3. N-carbamoylglutamate
4. N-carbamylglutamate
1. 1188-38-1
2. N-carbamyl-l-glutamic Acid
3. (s)-2-ureidopentanedioic Acid
4. Carbaglu
5. N-carbamylglutamate
6. N-carbamoyl-l-glutamic Acid
7. Ureidoglutaric Acid
8. Carbamylglutamic Acid
9. Carbamino-l-glutamic Acid
10. (2s)-2-(carbamoylamino)pentanedioic Acid
11. L-n-carbamoylglutamic Acid
12. N-carbamyl-l-glutamate
13. N-carbamyl-glutamic Acid
14. L-glutamic Acid, N-(aminocarbonyl)-
15. Oe-312
16. 5l0hb4v1ew
17. N-carbamoyl-l-glutamate
18. Chebi:71028
19. N-carbamoylglutamate
20. Oe 312
21. Nsc-760124
22. Oe 312 (laboratory Code Designation)
23. Dsstox_cid_26706
24. Dsstox_rid_81839
25. Dsstox_gsid_46706
26. Carglumic Acid [inn]
27. Carbamylglutamate
28. Carbaglu (tn)
29. Cas-1188-38-1
30. N-(aminocarbonyl)-l-glutamic Acid(carglumic Acid)
31. Unii-5l0hb4v1ew
32. Carglumic Acid [usan:inn]
33. Carglumic-acid
34. Acido Carglumico
35. Acide Carglumique
36. Acidum Carglumicum
37. Glutamic Acid, N-carbamoyl-, L-
38. Ncgc00167549-01
39. Carglumic Acid [mi]
40. Carglumic Acid [jan]
41. Carglumic Acid [usan]
42. Schembl373546
43. Carglumic Acid [vandf]
44. Gtpl7458
45. Carbamyl-l-glutamic Acid
46. Carglumic Acid [mart.]
47. Carglumic Acid [usp-rs]
48. Carglumic Acid [who-dd]
49. Chembl1201780
50. Dtxsid7046706
51. Carglumic Acid (jan/usan/inn)
52. Carglumic Acid [ema Epar]
53. Oe312
54. Bcp16753
55. Hy-b0711
56. Zinc1530283
57. Tox21_112544
58. Carglumic Acid [orange Book]
59. Mfcd00047874
60. S5301
61. Akos010384789
62. N-carbamyl-l-glutamic Acid Crystalline
63. Tox21_112544_1
64. Cs-8208
65. Db06775
66. Nsc 760124
67. Ncgc00274073-01
68. Ds-17934
69. C-1805
70. C05829
71. D07130
72. A804117
73. Q822884
74. J-507608
75. Z1551429737
76. Carglumic Acid, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 190.15 g/mol |
|---|---|
| Molecular Formula | C6H10N2O5 |
| XLogP3 | -2.4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 190.05897142 g/mol |
| Monoisotopic Mass | 190.05897142 g/mol |
| Topological Polar Surface Area | 130 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 227 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Carbaglu |
| PubMed Health | Carglumic acid (By mouth) |
| Drug Classes | Hyperammonemia Agent |
| Active Ingredient | Carglumic acid |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Prescription |
| Company | Orphan Europe |
| 2 of 2 | |
|---|---|
| Drug Name | Carbaglu |
| PubMed Health | Carglumic acid (By mouth) |
| Drug Classes | Hyperammonemia Agent |
| Active Ingredient | Carglumic acid |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Prescription |
| Company | Orphan Europe |
For the treatment of acute and chronic hyperammonaemia in patients with N-acetylglutamate synthase (NAGS) deficiency. This enzyme is an important component of the urea cycle to prevent build up of neurotoxic ammonium in the blood.
FDA Label
Carbaglu is indicated in treatment of:
- hyperammonaemia due to N-acetylglutamate-synthase primary deficiency;
- hyperammonaemia due to isovaleric acidaemia;
- hyperammonaemia due to methymalonic acidaemia;
- hyperammonaemia due to propionic acidaemia.
The median Tmax of Carbaglu was 3 hours (range: 2-4). The daily dose of carglumic acid ranges from 100 to 250 mg/kg and this does are normally adjusted to maintain normal plasma levels of ammonia.
A16AA05
A - Alimentary tract and metabolism
A16 - Other alimentary tract and metabolism products
A16A - Other alimentary tract and metabolism products
A16AA - Amino acids and derivatives
A16AA05 - Carglumic acid
Absorption
30% bioavailability; Cmax, mean, 100 mg/kg dose = 2.6 g/mL (range of 1.9 - 4.8) Carglumic acid is not subject to to intracellular degradation.
Route of Elimination
Following administration of a single radiolabeled oral dose of 100 mg/kg of body weight, 9% of the dose was excreted unchanged in the urine and up to 60% of the dose was excreted unchanged in the feces.
Volume of Distribution
The apparent volume of distribution was 2657 L (range: 1616-5797).
Clearance
The apparent total clearance was 5.7 L/min (range 3.0-9.7), the renal clearance was 290 mL/min (range 204-445), and the 24-hour urinary excretion was 4.5 % of the dose (range 3.5-7.5).
A proportion of carglumic acid may be metabolized by the intestinal bacterial flora. The likely end product of carglumic acid metabolism is carbon dioxide, eliminated through the lungs.
Median values for the terminal half-life was 5.6 hours (range 4.3-9.5).
Carglumic acid is a synthetic structural analogue of N-acetylglutamate (NAG), which is an essential allosteric activator of the liver enzyme carbamoyl phosphate synthetase 1 (CPS1). CPS1 is found in the mitochondria and is the first enzyme of the urea cycle, which converts ammonia into urea. Carglumic acid acts as a replacement for NAG in NAGS deficiency patients by activating CPS1 but it does not help to regulate the urea cycle.
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
GDUFA
DMF Review : Complete
Rev. Date : 2019-01-11
Pay. Date : 2018-12-14
DMF Number : 33166
Submission : 2018-12-07
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2024-05-06
Pay. Date : 2024-02-01
DMF Number : 39329
Submission : 2024-02-06
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2024-01-03
Pay. Date : 2023-09-27
DMF Number : 38477
Submission : 2023-07-12
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 26955
Submission : 2013-03-06
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?