
Synopsis
Synopsis
0
VMF
0
FDA Orange Book

0
Europe

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Aerosol Ot
2. Colace
3. Deh Na Ss
4. Deh-na-ss
5. Diethylhexyl Sodium Sulfosuccinate
6. Dioctyl Sulfosuccinate
7. Dioctyl Sulfosuccinate, Sodium
8. Dioctyl Sulfosuccinates
9. Dioctyl Sulfosuccinic Acid
10. Dioctyl Sulfosuccinic Acid, Ammonium Salt
11. Dioctyl Sulfosuccinic Acid, Barium Salt
12. Dioctyl Sulfosuccinic Acid, Calcium Salt
13. Dioctyl Sulfosuccinic Acid, Magnesium Salt
14. Dioctyl Sulfosuccinic Acid, Potassium Salt
15. Dioctyl Sulfosuccinic Acid, Sodium Salt
16. Dioctylsulfosuccinate
17. Dioctylsulphosuccinate, Sodium
18. Docusate
19. Docusate Calcium
20. Docusate Potassium
21. Doss
22. Sodium Bis(2-ethylhexyl)sulfosuccinate
23. Sodium Dioctyl Sulfosuccinate
24. Sodium Dioctylsulphosuccinate
25. Sodium Sulfosuccinate, Diethylhexyl
26. Sulfosuccinate, Diethylhexyl Sodium
27. Sulfosuccinate, Dioctyl
28. Sulfosuccinates, Dioctyl
29. Sulfosuccinic Acid Bis(2-ethylhexyl) Ester
1. 577-11-7
2. Dioctyl Sodium Sulfosuccinate
3. Dioctyl Sulfosuccinate Sodium Salt
4. Aerosol Ot
5. Constonate
6. Dioctylal
7. Diotilan
8. Disonate
9. Molatoc
10. Regutol
11. Doxol
12. Nevax
13. Velmol
14. Docusate (sodium)
15. Doxinate
16. Colace
17. Dialose
18. Molcer
19. Soliwax
20. Waxsol
21. Docusate Sodium Salt
22. Diox
23. Manoxol Ot
24. Adekacol Ec 8600
25. Bis(2-ethylhexyl) Sulfosuccinate Sodium Salt
26. Dioctyl Sulfosuccinate Sodium
27. Comfolax
28. Coprola
29. Dulcivac
30. Eurowet
31. Jamylene
32. Empimin Op70
33. Sanmorin Ot 70n
34. Airrol Ct-1
35. Mackanate Dos-70
36. Nikkol Otp-75
37. Triton Gr-pg 70
38. Gemtex Pa-70
39. Rapisol A 30
40. Bis(2-ethylhexyl) Sodium Sulfosuccinate
41. Nissan Rapisol A 30
42. Dioctyl Ester Of Sodium Sulfosuccinic Acid
43. Ins No.480
44. Monawet Mo 65-150
45. Sodium 1,4-bis[(2-ethylhexyl)oxy]-1,4-dioxobutane-2-sulfonate
46. Ins-480
47. Butanedioic Acid, Sulfo-, 1,4-bis(2-ethylhexyl) Ester, Sodium Salt
48. F05q2t2ja0
49. Sodium 1,4-bis(2-ethylhexyl) Sulfosuccinate
50. Diethylhexyl Sodium Sulfosuccinate
51. Sodium Docusate
52. Nsc-760404
53. Aot
54. Diomedicone
55. Clestol
56. Complemix
57. Defilin
58. Dioctlyn
59. Diosuccin
60. Dulsivac
61. Laxinate
62. Mervamine
63. Molofac
64. Requtol
65. Sobital
66. Coprol
67. Diovac
68. Konlax
69. Kosate
70. Obston
71. Softil
72. Revac
73. E 480
74. E-480
75. Modane Soft
76. Dsstox_cid_2959
77. Alcopol O
78. Alphasol Ot
79. Manoxal Ot
80. Sulfimel Dos
81. Aerosol Aot
82. Aerosol Gpg
83. Wetaid Sr
84. Dsstox_rid_76808
85. Aerosol Ot-a
86. Dsstox_gsid_22959
87. Laxinate 100
88. Sanmorin Ot 70
89. Triton Gr 7
90. Triton Gr-5
91. Aerosol Ot 70pg
92. Aerosol Ot 75
93. Celanol Dos 65
94. Celanol Dos 75
95. Dess
96. Humifen Wt 27g
97. Monawet Md 70e
98. Solusol-75%
99. Nikkol Otp 70
100. Aerosol A 501
101. Alkasurf Ss-o 75
102. Solusol-100%
103. Nekal Wt-27
104. Berol 478
105. Coloxyl
106. Dioctyl
107. Docolace
108. Docuprene
109. Geriplex
110. Silace
111. Unilax
112. Bloat Treatment
113. Docusato Sodico
114. Sodium Bis(2-ethylhexyl) Sulfosuccinate
115. Dioctyl Sodium Sulfosuccinate (jan)
116. Docusate Sodique
117. Dialose Plus
118. Natrii Dioctylsulfosuccinas
119. Tex-wet 1001
120. Senokot S
121. Correctol Caplets
122. Correctol Tablets
123. Senokap Dss
124. Docusatum Natricum
125. D-s-s
126. Doc Q Lace
127. Cas-577-11-7
128. Sodium Dioctyl Sulphosuccinate
129. Feen-a-mint Pills
130. Dioctyl Sodium Sulfosuccinate [jan]
131. Correctol Extra Gentle Tablets
132. Docusate Sodique [inn-french]
133. Docusato Sodico [inn-spanish]
134. Docusatum Natricum [inn-latin]
135. Hsdb 3065
136. 2-ethylhexyl Sulfosuccinate Sodium
137. Ncgc00164140-03
138. Einecs 209-406-4
139. Sv 102
140. Bis(2-ethylhexyl)sodium Sulfosuccinate
141. Dioctyl Ester Of Sodium Sulfosuccinate
142. Di-(2-ethylhexyl) Sodium Sulfosuccinate
143. Sodium Di-(2-ethylhexyl) Sulfosuccinate
144. Dioctyl Sodium Sulphosuccinat
145. Unii-f05q2t2ja0
146. Prenexa
147. Purgasol
148. Vinacol
149. Bis(2-ethylhexyl) S-sodium Sulfosuccinate
150. Ai3-00239
151. Docusate Sod
152. Sodium 1,4-bis(2-ethylhexyl)sulfosuccinate
153. 1,4-bis(2-ethylhexyl) Sodium Sulfosuccinate
154. Sodium Sulfodi-(2-ethylhexyl)-sulfosuccinate
155. Senexon-s
156. Folca[s Care Pme
157. Folcal Dha
158. Senna-s
159. Di(2-ethylhexyl)sulfosuccinic Acid, Sodium Salt
160. Bis(ethylhexyl) Ester Of Sodium Sulfosuccinic Acid
161. Bis-2-ethylhexylester Sulfojantaranu Sodneho [czech]
162. Colace (tn)
163. Mfcd00012455
164. Sodium Di(2-ethylhexyl)sulfosuccinate
165. Sulfosuccinic Acid, Bis(2-ethylhexyl)ester Sodium Salt
166. Docusate Sodium [usan:usp:inn:ban]
167. Sol Sodowej Sulfobursztynianu Dwu-2-etyloheksylowego [polish]
168. Docusate Sodium (usp)
169. Docusate Sodium Solution
170. Bis-2-ethylhexylester Sulfojantaranu Sodneho
171. Chemax Doss/75e
172. Succinic Acid, Sulfo-, 1,4-bis(2-ethylhexyl) Ester, Sodium Salt
173. Ncgc00183136-01
174. Ec 209-406-4
175. Schembl4113
176. Docusate Sodium [ii]
177. Docusate Sodium [mi]
178. Mls004773938
179. Docusate Sodium [inn]
180. 1,4-bis(2-ethylhexyl)sulfobutanedioate, Sodium Salt
181. Sol Sodowej Sulfobursztynianu Dwu-2-etyloheksylowego
182. Docusate Sodium [hsdb]
183. Docusate Sodium [usan]
184. Chebi:4674
185. Sulfosuccinic Acid Bis(2-ethylhexyl) Ester Sodium Salt
186. Docusate Sodium [vandf]
187. Chembl1905872
188. Docusate Sodium [mart.]
189. Dtxsid8022959
190. Correctol Stool Softener Laxative
191. Docusate Sodium [usp-rs]
192. Docusate Sodium [who-dd]
193. Sulfosuccinic Acid, Di-(2-ethylhexyl) Ester, Sodium Salt
194. Hms3264p07
195. Hms3885b10
196. Sodium;1,4-bis(2-ethylhexoxy)-1,4-dioxobutane-2-sulfonate
197. Bcp31325
198. Hy-b1268
199. 4-(4-bromophenoxymethyl)benzoicacid
200. Aerosol™ Ot, Solid Anhydrous
201. Sodium Dioctyl Sulfosuccinate (inn)
202. Tox21_112087
203. Tox21_113469
204. Tox21_201342
205. Tox21_300496
206. S4588
207. Dioctyl Disodium Sulfosuccinate
208. Akos015901806
209. Docusate Sodium [usp Impurity]
210. Ccg-213234
211. Cs-4813
212. Docusate Sodium [usp Monograph]
213. Nsc 760404
214. Sodium Di(2-ethylhexyl) Sulfosuccinate
215. Docusate Sodium Salt, Bioxtra, >=99%
216. Docusate Sodium Solution [vandf]
217. Dioctyl Sulfosuccinate Sodium Salt, 96%
218. Ncgc00164140-01
219. Ncgc00254414-01
220. Ncgc00258894-01
221. As-13347
222. E480
223. Smr001595510
224. Aerosol™ Ot Solution, 10% (w/w)
225. Dioctyl Sodium Sulfosuccinate [fcc]
226. Dioctyl Sodium Sulfosuccinate With Ethanol
227. Bis(2-ethylhexyl) Sulfosuccinatesodium Salt
228. Dioctyl Sulfosuccinate Sodium Salt, >=97%
229. Aec Diethylhexyl Sodium Sulfosuccinate
230. Ft-0689234
231. D00305
232. Docusate Sodium Salt, P.a., 99.0-100.5%
233. E77584
234. Diethylhexyl Sodium Sulfosuccinate [inci]
235. Docusate Sodium Salt, Purum, >=96.0% (tlc)
236. Docusate Sodium Salt, Bioultra, >=99.0% (tlc)
237. Docusate Sodium, Meets Usp Testing Specifications
238. Dioctyl Sodium Sulfosuccinate With Diethylene Glycol
239. Dioctyl Sodium Sulfosuccinate With Propylene Glycol
240. Q2815334
241. W-105447
242. F8880-5559
243. Docusate Sodium, British Pharmacopoeia (bp) Reference Standard
244. Docusate Sodium, European Pharmacopoeia (ep) Reference Standard
245. Sodium 1,4-bis(2-ethylhexyloxy)-1,4-dioxobutane-2-sulfonate
246. Docusate Sodium, United States Pharmacopeia (usp) Reference Standard
247. Dioctyl Sodium Sulfosuccinate With Diethylene Glycol And Glycol Ethers
248. 1,4-bis(2-ethylhexyl)sodiumsulfosuccinate Pound>>dioctyl Sulfosuccinate Sodium Salt
249. Butanedioic Acid, 2-sulfo-, 1,4-bis(2-ethylhexyl) Ester, Sodium Salt (1:1)
250. Yal
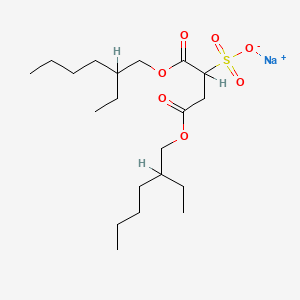
| Molecular Weight | 444.6 g/mol |
|---|---|
| Molecular Formula | C20H37NaO7S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 18 |
| Exact Mass | 444.21576897 g/mol |
| Monoisotopic Mass | 444.21576897 g/mol |
| Topological Polar Surface Area | 118 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 546 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 3 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Cathartics; Excipients; Surface-Active Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Oral bulk-forming, lubricant, and stool softener laxatives are indicated prophylactically in patients who should not strain during defecation, such as those with an episiotomy wound, painful thrombosed hemorrhoids, fissures or perianal abbesses, body wall and diaphragmatic hernias, anorectal stenosis, or postmyocardial infarction. /Laxatives; Included in US product labeling/
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1704
Oral laxatives are indicated for the short-term relief of constipation. Oral bulk-forming laxatives, stimulant laxatives, and carbon dioxide-releasing suppositories are indicated to facilitate defecation in geriatric patients with diminished colonic motor response. Oral bulk-forming laxatives and stool softener laxatives are preferred to treat constipation that may occur during pregnancy and postpartum to help re-establish normal bowel function or to avoid straining if hemorrhoids are present. In severe cases of constipation, such as with fecal impaction, mineral oil and stool softener laxatives administered orally or rectally are indicated to soften the impacted feces. To help complete the evacuation of the impacted colon, a rectal stimulant or saline laxative may follow. /Laxatives; Included in US product labeling/
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1704
Laxatives should not be given to young children (up to 6 years of age) unless prescribed by a physician. Since children are not usually able to describe their symptoms precisely, proper diagnosis should precede the use of a laxative. This will avoid the complication of an existing condition(e.g., appendicitis) or the appearance of more severe side effects. /Laxatives/
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1705
Side/Adverse Effects: Those indicating need for medical attention: Incidence rare: Allergies, undetermined (skin rash). /Laxatives/
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1707
Side/Adverse Effects: Those indicating need for medical attention only if they continue or are bothersome: Incidence less frequent: Stomach and/or intestinal cramping; throat irritation - with liquid forms. /Laxatives/
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1707
Because concurrent use of the docusate salts may increase the absorption of mineral oil, their concomitant administration is not recommended. /Docusate salts/
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 952
For more Drug Warnings (Complete) data for BIS(2-ETHYLHEXYL) SODIUM SULFOSUCCINATE (7 total), please visit the HSDB record page.
3. 3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG, BETWEEN 1 OZ & 1 PINT FOR 70 KG PERSON (150 LB).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-274
Surface-Active Agents
Agents that modify interfacial tension of water; usually substances that have one lipophilic and one hydrophilic group in the molecule; includes soaps, detergents, emulsifiers, dispersing and wetting agents, and several groups of antiseptics. (See all compounds classified as Surface-Active Agents.)
A06AA02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AA - Softeners, emollients
A06AA02 - Docusate sodium
...DRUG IS ABSORBED FROM GI TRACT & IS EXCRETED IN SIGNIFICANT CONCN IN BILE.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 922
SINCE THE DRUG IS ABSORBED FROM GI TRACT & IS EXCRETED IN SIGNIFICANT CONCN IN BILE...
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 922
In vitro studies suggest that these salts of dioctylsulfosuccinic acid lower the surface tension of the stool to permit water and lipids to enter more readily and thus soften the feces. ... More recent evidence indicates that they may stimulate the secretion of water and electrolytes on contact with the mucosa.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 949
Reduce surface film tension of interfacing liquid contents of the bowel, promoting permeation of additional liquid into the stool to form a softer mass. /Laxatives/
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1705
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3235
Submission : 1978-06-22
Status : Active
Type : IV

Certificate Number : R1-CEP 2015-208 - Rev 00
Issue Date : 2022-05-23
Type : Chemical
Substance Number : 1418
Status : Valid

Registration Number : 218MF10083
Registrant's Address : 504 Carnegie Center, Princeton, NJ 08540, U.S. S. A.
Initial Date of Registration : 2006-01-27
Latest Date of Registration :

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2972
Submission : 1977-06-15
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3246
Submission : 1978-06-16
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3106
Submission : 1978-01-06
Status : Inactive
Type : II



Date of Issue : 2021-12-09
Valid Till : 2024-12-08
Written Confirmation Number : WC-0515
Address of the Firm :


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
40
PharmaCompass offers a list of Docusate Sodium API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Docusate Sodium manufacturer or Docusate Sodium supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Docusate Sodium manufacturer or Docusate Sodium supplier.
PharmaCompass also assists you with knowing the Docusate Sodium API Price utilized in the formulation of products. Docusate Sodium API Price is not always fixed or binding as the Docusate Sodium Price is obtained through a variety of data sources. The Docusate Sodium Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A CAS-577-11-7 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of CAS-577-11-7, including repackagers and relabelers. The FDA regulates CAS-577-11-7 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. CAS-577-11-7 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of CAS-577-11-7 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A CAS-577-11-7 supplier is an individual or a company that provides CAS-577-11-7 active pharmaceutical ingredient (API) or CAS-577-11-7 finished formulations upon request. The CAS-577-11-7 suppliers may include CAS-577-11-7 API manufacturers, exporters, distributors and traders.
click here to find a list of CAS-577-11-7 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A CAS-577-11-7 DMF (Drug Master File) is a document detailing the whole manufacturing process of CAS-577-11-7 active pharmaceutical ingredient (API) in detail. Different forms of CAS-577-11-7 DMFs exist exist since differing nations have different regulations, such as CAS-577-11-7 USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A CAS-577-11-7 DMF submitted to regulatory agencies in the US is known as a USDMF. CAS-577-11-7 USDMF includes data on CAS-577-11-7's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The CAS-577-11-7 USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of CAS-577-11-7 suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The CAS-577-11-7 Drug Master File in Japan (CAS-577-11-7 JDMF) empowers CAS-577-11-7 API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the CAS-577-11-7 JDMF during the approval evaluation for pharmaceutical products. At the time of CAS-577-11-7 JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of CAS-577-11-7 suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a CAS-577-11-7 Drug Master File in Korea (CAS-577-11-7 KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of CAS-577-11-7. The MFDS reviews the CAS-577-11-7 KDMF as part of the drug registration process and uses the information provided in the CAS-577-11-7 KDMF to evaluate the safety and efficacy of the drug.
After submitting a CAS-577-11-7 KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their CAS-577-11-7 API can apply through the Korea Drug Master File (KDMF).
click here to find a list of CAS-577-11-7 suppliers with KDMF on PharmaCompass.
A CAS-577-11-7 CEP of the European Pharmacopoeia monograph is often referred to as a CAS-577-11-7 Certificate of Suitability (COS). The purpose of a CAS-577-11-7 CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of CAS-577-11-7 EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of CAS-577-11-7 to their clients by showing that a CAS-577-11-7 CEP has been issued for it. The manufacturer submits a CAS-577-11-7 CEP (COS) as part of the market authorization procedure, and it takes on the role of a CAS-577-11-7 CEP holder for the record. Additionally, the data presented in the CAS-577-11-7 CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the CAS-577-11-7 DMF.
A CAS-577-11-7 CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. CAS-577-11-7 CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of CAS-577-11-7 suppliers with CEP (COS) on PharmaCompass.
A CAS-577-11-7 written confirmation (CAS-577-11-7 WC) is an official document issued by a regulatory agency to a CAS-577-11-7 manufacturer, verifying that the manufacturing facility of a CAS-577-11-7 active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting CAS-577-11-7 APIs or CAS-577-11-7 finished pharmaceutical products to another nation, regulatory agencies frequently require a CAS-577-11-7 WC (written confirmation) as part of the regulatory process.
click here to find a list of CAS-577-11-7 suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing CAS-577-11-7 as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for CAS-577-11-7 API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture CAS-577-11-7 as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain CAS-577-11-7 and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a CAS-577-11-7 NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of CAS-577-11-7 suppliers with NDC on PharmaCompass.
CAS-577-11-7 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of CAS-577-11-7 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right CAS-577-11-7 GMP manufacturer or CAS-577-11-7 GMP API supplier for your needs.
A CAS-577-11-7 CoA (Certificate of Analysis) is a formal document that attests to CAS-577-11-7's compliance with CAS-577-11-7 specifications and serves as a tool for batch-level quality control.
CAS-577-11-7 CoA mostly includes findings from lab analyses of a specific batch. For each CAS-577-11-7 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
CAS-577-11-7 may be tested according to a variety of international standards, such as European Pharmacopoeia (CAS-577-11-7 EP), CAS-577-11-7 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (CAS-577-11-7 USP).
