
Synopsis
Synopsis
0
VMF
0
Australia

US Medicaid
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
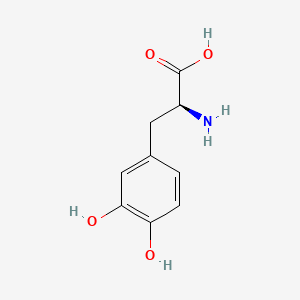

1. 3 Hydroxy L Tyrosine
2. 3-hydroxy-l-tyrosine
3. Dopaflex
4. Dopar
5. L 3,4 Dihydroxyphenylalanine
6. L Dopa
7. L-3,4-dihydroxyphenylalanine
8. L-dopa
9. Larodopa
10. Levopa
1. L-dopa
2. 59-92-7
3. Dopar
4. 3,4-dihydroxy-l-phenylalanine
5. 3-hydroxy-l-tyrosine
6. Bendopa
7. Larodopa
8. Levopa
9. 3,4-dihydroxyphenylalanine
10. Brocadopa
11. Cidandopa
12. Insulamina
13. Maipedopa
14. Dopaidan
15. Dopalina
16. Dopasol
17. Eldopal
18. Eldopar
19. Pardopa
20. Prodopa
21. Syndopa
22. 3-(3,4-dihydroxyphenyl)-l-alanine
23. (-)-dopa
24. Dihydroxy-l-phenylalanine
25. Helfo-dopa
26. Dopaflex
27. Deadopa
28. Dopal-fher
29. Doparkine
30. Dopaston
31. Dopastral
32. Eldopatec
33. Eurodopa
34. Doparl
35. Doprin
36. Veldopa
37. L-3,4-dihydroxyphenylalanine
38. (2s)-2-amino-3-(3,4-dihydroxyphenyl)propanoic Acid
39. Levedopa
40. Levodopum
41. L-o-hydroxytyrosine
42. L-tyrosine, 3-hydroxy-
43. Dopa
44. (-)-3-(3,4-dihydroxyphenyl)-l-alanine
45. Ledopa
46. 3,4-dihydroxyphenyl-l-alanine
47. Dopaston Se
48. Beta-(3,4-dihydroxyphenyl)-l-alanine
49. L-(o-dihydroxyphenyl)alanine
50. L-(-)-dopa
51. L-3-hydroxytyrosine
52. L-beta-(3,4-dihydroxyphenyl)alanine
53. Weldopa
54. Parda
55. L-dihydroxyphenylalanine
56. L-3-(3,4-dihydroxyphenyl)alanine
57. (s)-2-amino-3-(3,4-dihydroxyphenyl)propanoic Acid
58. Ro 4-6316
59. Beta-(3,4-dihydroxyphenyl)alanine
60. Inbrija
61. Cvt-301
62. Alanine, 3-(3,4-dihydroxyphenyl)-, L-
63. Dihydroxyphenylalanine
64. Component Of Sinemet
65. Dopar (tn)
66. Chebi:15765
67. Beta-(3,4-dihydroxyphenyl)-alpha-l-alanine
68. L-beta-(3,4-dihydroxyphenyl)-alpha-alanine
69. Alanine, 3-(3,4-dihydroxyphenyl)-, (-)-
70. L(-)-dopa
71. (-)-(3,4-dihydroxyphenyl)alanine
72. Nsc-118381
73. L-.beta.-(3,4-dihydroxyphenyl)alanine
74. Chembl1009
75. .beta.-(3,4-dihydroxyphenyl)-l-alanine
76. L-(3,4-dihydroxyphenyl)alanine
77. L-tyrosine, 3-hydroxy-, Homopolymer
78. Nsc118381
79. .beta.-(3,4-dihydroxyphenyl)alanine
80. Cas-59-92-7
81. Ncgc00016270-04
82. Biodopa
83. Cerepap
84. Laradopa
85. Sobiodopa
86. L-(3,4-dihydroxyphenyl)-.alpha.-alanine
87. 46627o600j
88. Mfcd00002598
89. V-1512
90. C9h11no4
91. Helfo Dopa
92. 65170-01-6
93. Beta-(3,4-dihydroxyphenyl)-alpha-alanine
94. Atamet
95. Levodopum [inn-latin]
96. Bdbm50130192
97. L-o-dihydroxyphenylalanine
98. L Dopa
99. L-3
100. Ccris 3766
101. Hsdb 3348
102. Wln: Qvyz1r Cq Dq
103. 3,4-dihydroxyphenylalanine (van)
104. Sr-01000075384
105. Einecs 200-445-2
106. Nsc 118381
107. L-3,4-dihydrophenylalanine
108. Dopastone
109. Dopicar
110. Prolopa
111. (2s)-2-amino-3-(3,4-dihydroxyphenyl)propanoate
112. Unii-46627o600j
113. Prestwick_185
114. Levodopa (sinemet)
115. L-dopa; Levodopa
116. Madopa (salt/mix)
117. Levodopa [usan:usp:inn:ban:jan]
118. Spectrum_000454
119. 4-dihydroxyphenylalanine
120. Levodopa [hsdb]
121. Levodopa [usan]
122. Levodopa [inn]
123. Levodopa [jan]
124. Carbidopa Ep Impurity A
125. Levodopa [mi]
126. Levodopa [vandf]
127. Prestwick0_000017
128. Prestwick1_000017
129. Prestwick2_000017
130. Prestwick3_000017
131. Spectrum2_000496
132. Spectrum4_000539
133. Spectrum5_001899
134. Lopac-d-9628
135. Levodopa (jp15/usp)
136. Dsstox_cid_3209
137. Levodopa [mart.]
138. Bmse000322
139. Epitope Id:150927
140. Levodopa [usp-rs]
141. Levodopa [who-dd]
142. Levodopa [who-ip]
143. Dopa, L-
144. 3, 4-dihydroxyphenylalanine
145. Alanine,4-dihydroxyphenyl)-
146. Dsstox_rid_76926
147. Levodopa [ema Epar]
148. Dsstox_gsid_23209
149. Lopac0_000454
150. Schembl22655
151. Bspbio_000053
152. Bspbio_002354
153. Kbiogr_001177
154. Kbioss_000934
155. L-4-5-dihydroxyphenylalanine
156. Mls000028514
157. Bidd:gt0158
158. Divk1c_000452
159. Spectrum2300205
160. Levodopa (jp17/usp/inn)
161. Spbio_000391
162. Spbio_001974
163. Dhivy Component Levodopa
164. Duopa Component Levodopa
165. Levodopa [ep Impurity]
166. Levodopa [orange Book]
167. Bpbio1_000059
168. Gtpl3639
169. Levodopa [ep Monograph]
170. Rytary Component Levodopa
171. B-(3,4-dihydroxyphenyl)alanine
172. Dtxsid9023209
173. Levodopa [usp Monograph]
174. Wln: Qvyz1r Cq Dq -l
175. 3, 4-dihydroxy-l-phenylalanine
176. Bdbm60928
177. Hms501g14
178. Kbio1_000452
179. Kbio2_000934
180. Kbio2_003502
181. Kbio2_006070
182. Parcopa Component Levodopa
183. Stalevo Component Levodopa
184. Levodopum [who-ip Latin]
185. Alanine,4-dihydroxyphenyl)-, L-
186. Carbilev Component Levodopa
187. Corbilta Component Levodopa
188. Dopasnap Component Levodopa
189. Ipx203 Component Levodopa
190. L-(3, 4-dihydroxyphenyl)alanine
191. Ninds_000452
192. Hms1568c15
193. Hms1922j14
194. Hms2090o08
195. Hms2093n04
196. Hms2095c15
197. Hms2230b04
198. Hms3261k10
199. Hms3712c15
200. Levodopa Component Of Dhivy
201. Levodopa Component Of Duopa
202. Pharmakon1600-02300205
203. Zinc895199
204. H-phe{3,4-(oh)2}-oh
205. Hy-n0304
206. Ipx-203 Component Levodopa
207. Levodopa Component Of Rytary
208. Levodopa;3,4-dihydroxyphenylalanine
209. B-(3,4-dihydroxyphenyl)-l-alanine
210. Inbrija (levodopa Inhalation Powder)
211. Levodopa Component Of Parcopa
212. Levodopa Component Of Sinemet
213. Levodopa Component Of Stalevo
214. Tox21_110338
215. Tox21_500454
216. Ccg-39571
217. L-3-(3,4-dihydroxy-phenyl)alanine
218. L-3-(3,4-dihydroxyphenyl)-alanine
219. Nsc759573
220. Pdsp1_001541
221. Pdsp2_001525
222. S1726
223. Alanine, 3-(3,4-dihydroxyphenyl)-
224. Alanine,4-dihydroxyphenyl)-, (-)-
225. Levodopa Component Of Carbilev
226. Levodopa Component Of Corbilta
227. Levodopa Component Of Dopasnap
228. Akos010396267
229. B-(3,4-dihydroxyphenyl)-a-l-alanine
230. L-b-(3,4-dihydroxyphenyl)-a-alanine
231. .beta.-(3, 4-dihydroxyphenyl)alanine
232. Ac-8432
233. Am82124
234. Cs-1945
235. Db01235
236. Lp00454
237. Nsc-759573
238. Sdccgmls-0066924.p001
239. Sdccgsbi-0050439.p004
240. Idi1_000452
241. Ncgc00015384-01
242. Ncgc00016270-01
243. Ncgc00016270-06
244. Ncgc00016270-07
245. Ncgc00016270-09
246. Ncgc00016270-10
247. Ncgc00016270-22
248. Ncgc00093869-04
249. Ncgc00261139-01
250. As-13287
251. Bp-12850
252. Smr000058312
253. Sbi-0050439.p003
254. L-(3, 4-dihydroxyphenyl)-.alpha.-alanine
255. D0600
256. D9628
257. Eu-0100454
258. N1648
259. 59l927
260. Alanine, 3-(3, 4-dihydroxyphenyl)-, (-)-
261. C00355
262. D 9628
263. D00059
264. D70595
265. 3,4-dihydroxy-l-phenylalanine, >=98% (tlc)
266. Ab00052418-06
267. Ab00052418-07
268. Ab00052418_08
269. Ab00052418_09
270. A832543
271. Q300989
272. Q-201294
273. Sr-01000075384-1
274. Sr-01000075384-4
275. Sr-01000075384-6
276. Sr-01000075384-7
277. (s)-2-amino-3-(3,4-dihydroxy-phenyl)-propionic Acid
278. F0347-4695
279. Levodopa, British Pharmacopoeia (bp) Reference Standard
280. Levodopa, European Pharmacopoeia (ep) Reference Standard
281. Z1762772338
282. (2s)-2-amino-3-(3,4-dihydroxyphenyl)propanoic Acidl-dopa
283. 1e83f927-c221-46aa-b90a-81b33c5f3868
284. 3,4-dihydroxy-l-phenylalanine, Vetec(tm) Reagent Grade, 98%
285. Levodopa Component Of Levodopa/carbidopa/entacapone Orion
286. Levodopa, United States Pharmacopeia (usp) Reference Standard
287. Levodopa/carbidopa/entacapone Orion Component Levodopa
288. 3,4-dihydroxy-l-phenylalanine, Certified Reference Material, Tracecert(r)
289. Levodopa, Pharmaceutical Secondary Standard; Certified Reference Material
290. 122769-74-8
291. L-methyldopa ; (2s)-2-amino-3-(3,4-dihydroxyphenyl)-2-methylpropanoic Acid; 3-(3,4-dihydroxyphenyl)-?-methyl-l-alanine; 3-hydroxy-a-methyl-l-tyrosine
| Molecular Weight | 197.19 g/mol |
|---|---|
| Molecular Formula | C9H11NO4 |
| XLogP3 | -2.7 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 197.06880783 g/mol |
| Monoisotopic Mass | 197.06880783 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 209 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Levodopa is indicated to alleviate symptoms and allow more normal body movements with improved muscle control in the treatment of idiopathic Parkinson's disease, postencephalitic parkinsonism, or symptomatic parkinsonism that may follow injury to the nervous system by carbon monoxide intoxication or manganese intoxication. It is also indicated in parkinsonism associated with cerebral arteriosclerosis. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional 21 st ed. Volume 1. MICROMEDEX Thomson Health Care, Englewood, CO. 2001. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1902
Levodopa, the metabolic precursor of dopamine, is the single most effective agent in the treatment of Parkinson's Disease.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 555
LEVODOPA AFFORDS ONLY SYMPTOMATIC RELIEF OF PARKINSONISM. IF DRUG IS STOPPED, ALL PREEXISTING SYMPTOMS GRADUALLY RETURN WITHIN WK OR TWO; UPON RESUMPTION OF L-DOPA THERAPY AFTER PERIOD OF WITHDRAWAL, PREVIOUS THERAPEUTIC RESPONSE IS REESTABLISHED AFTER WK OR MORE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 235
...L-DOPA...HAS BEEN MORE CONSISTENTLY EFFECTIVE IN TREATMENT OF CHRONIC MANGANESE POISONING THAN IN PARKINSON'S DISEASE.
Doull, J., C.D. Klaassen, and M. D. Amdur (eds.). Casarett and Doull's Toxicology. 2nd ed. New York: Macmillan Publishing Co., 1980., p. 450
For more Therapeutic Uses (Complete) data for LEVODOPA (17 total), please visit the HSDB record page.
PT WITH CARDIAC ARRHYTHMIAS OR HISTORY OF MYOCARDIAL INFARCTION SHOULD UNDERGO INITIAL THERAPY WITH LEVODOPA ONLY IN FACILITY EQUIPPED FOR INTENSIVE CORONARY CARE. ...DIABETIC PT...SHOULD BE CAREFULLY MONITORED FOR ANY NECESSITY TO MODIFY THEIR REGIMEN. CAUTION ALSO NECESSARY IN PT WITH HISTORY OF PEPTIC ULCER, CONVULSIONS...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 233
DISCONTINUATION OF LEVODOPA THERAPY FOR 6-24 HR PRIOR TO GENERAL ANESTHESIA IS RECOMMENDED... SAFETY OF L-DOPA DURING PREGNANCY HAS NOT BEEN ESTABLISHED, HOWEVER, INFANTS SHOULD NOT BE NURSED BY MOTHERS RECEIVING DRUG SINCE IT MAY APPEAR IN MILK. ...DRUG MAY INHIBIT LACTATION.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 233
...IS CONTRAINDICATED IN PATIENTS WITH NARROW-ANGLE GLAUCOMA, ACUTE PSYCHOSIS, OR SEVERE PSYCHONEUROSIS. IT SHOULD NOT BE ADMIN TO PT WITH UNCOMPENSATED ENDOCRINE, RENAL, HEPATIC, CARDIOVASCULAR, OR PULMONARY DISEASE. /USE/...EXTREME CAUTION IN PT WITH ASTHMA OR EMPHYSEMA WHO MAY REQUIRE SYMPATHOMIMETIC DRUGS.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 233
SINCE LEVODOPA THERAPY...ASSOC WITH INCR GROWTH OF MELANOMA, PT WITH KNOWN MELANOMAS OR PIGMENTED LESIONS SHOULD BE CAREFULLY MONITORED FOR CHANGES IN SUCH LESIONS & DRUG WITHDRAWN IF CHANGES OCCUR.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 233
For more Drug Warnings (Complete) data for LEVODOPA (49 total), please visit the HSDB record page.
Levodopa on its own is formulated as an oral inhalation powder indicated for intermittent treatment of off episodes in Parkinson's patients who are already being treated with carbidopa and levodopa. Levodopa is most commonly formulated as an oral tablet with a peripheral dopa decarboxylase inhibitor indicated for treatment of Parkinson's disease, post-encephalitic parkinsonism, and symptomatic parkinsonism following carbon monoxide intoxication or manganese intoxication.
FDA Label
Inbrija is indicated for the intermittent treatment of episodic motor fluctuations (OFF episodes) in adult patients with Parkinsons disease (PD) treated with a levodopa/dopa-decarboxylase inhibitor.
Levodopa is able to cross the blood-brain barrier while dopamine is not. The addition of a peripheral dopa decarboxylase inhibitor prevents the conversion of levodopa to dopamine in the periphery so that more levodopa can reach the blood-brain barrier. Once past the blood-brain barrier, levodopa is converted to dopamine by aromatic-L-amino-acid decarboxylase.
Antiparkinson Agents
Agents used in the treatment of Parkinson's disease. The most commonly used drugs act on the dopaminergic system in the striatum and basal ganglia or are centrally acting muscarinic antagonists. (See all compounds classified as Antiparkinson Agents.)
Dopamine Agents
Any drugs that are used for their effects on dopamine receptors, on the life cycle of dopamine, or on the survival of dopaminergic neurons. (See all compounds classified as Dopamine Agents.)
N04BA01
N04BA02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N04 - Anti-parkinson drugs
N04B - Dopaminergic agents
N04BA - Dopa and dopa derivatives
N04BA01 - Levodopa
Absorption
Orally inhaled levodopa reaches a peak concentration in 0.5 hours with a bioavailability than is 70% that of the immediate release levodopa tablets with a peripheral dopa decarboxylase inhibitor like carbidopa or benserazide.
Route of Elimination
After 48 hours, 0.17% of an orally administered dose is recovered in stool, 0.28% is exhaled, and 78.4% is recovered in urine
Volume of Distribution
168L for orally inhaled levodopa.
Clearance
Intravenously administered levodopa is cleared at a rate of 14.2mL/min/kg in elderly patients and 23.4mL/min/kg in younger patients. When given carbidopa, the clearance of levodopa was 5.8mL/min/kg in elderyly patients and 9.3mL/min/kg in younger patients.
...DRUG...MAY APPEAR IN MILK.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 233
AFTER IP INJECTION INTO MICE, BIOTRANSFORMATION OF 60% OF RADIOACTIVELY LABELLED DL-DOPA TAKES PLACE WITHIN 10 MIN, & PEAK DOPAMINE LEVELS ARE REACHED 20 MIN AFTER ADMIN. ...APPROX 0.1% OF DOSE WAS PRESENT IN THE BRAIN AS (14)C-L-DOPA OR (14)C-DOPAMINE. /DL-DOPA/
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 213
MORE THAN 95% OF LEVODOPA IS DECARBOXYLATED IN PERIPHERY BY WIDELY DISTRIBUTED EXTRACEREBRAL AROMATIC L-AMINO ACID DECARBOXYLASE. ...LITTLE UNCHANGED DRUG REACHES CEREBRAL CIRCULATION & PROBABLY LESS THAN 1% PENETRATES INTO CNS.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 230
MOST IS CONVERTED TO DOPAMINE... DOPAMINE METABOLITES ARE RAPIDLY EXCRETED IN URINE, ABOUT 80% OF RADIOACTIVELY LABELED DOSE BEING RECOVERED WITHIN 24 HR. ... THESE METABOLITES /3,4-DIHYDROXYPHENYLACETIC ACID & 3-METHOXY-4-HYDROXYPHENYLACETIC ACID/, AS WELL AS SMALL AMT OF LEVODOPA & DOPAMINE, ALSO APPEAR IN CEREBROSPINAL FLUID.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 231
For more Absorption, Distribution and Excretion (Complete) data for LEVODOPA (11 total), please visit the HSDB record page.
Levodopa is either converted to dopamine by aromatic-L-amino-acid decarboxylase or O-methylated to 3-O-methyldopa by catechol-O-methyltransferase. 3-O-methyldopa cannot be metabolized to dopamine. Once levodopa is converted to dopamine, it is converted to sulfated or glucuronidated metabolites, epinephrine E, or homovanillic acid through various metabolic processes. The primary metabolites are 3,4-dihydroxyphenylacetic acid (13-47%) and homovanillic acid (23-39%).
MOST IS CONVERTED TO DOPAMINE... BIOTRANSFORMATION OF DOPAMINE PROCEEDS RAPIDLY...EXCRETION PRODUCTS, 3,4-DIHYDROXYPHENYLACETIC ACID...& 3-METHOXY-4-HYDROXYPHENYLACETIC ACID... SOME BIOCHEMICAL EVIDENCE INDICATES THAT ACCELERATION OF LEVODOPA METABOLISM OCCURS DURING PROLONGED THERAPY, POSSIBLY DUE TO ENZYME INDUCTION.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 230
MORE THAN 95%...IS DECARBOXYLATED...BY...AROMATIC L-AMINO ACID DECARBOXYLASE. ... A SMALL AMT /OF L-DOPA/ IS METHYLATED TO 3-O-METHYL-DOPA... MOST IS CONVERTED TO DOPAMINE, SMALL AMT OF WHICH IN TURN ARE METABOLIZED TO NOREPINEPHRINE & EPINEPHRINE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 230
...IS ESTIMATED THAT ABOUT THREE FOURTHS OF DIETARY METHIONINE IS UTILIZED FOR METABOLISM OF LARGE THERAPEUTIC DOSES OF LEVODOPA.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 231
LEVODOPA (L-DOPA) IS FORMED IN MAMMALS FROM L-TYROSINE AS INTERMEDIARY METABOLITE IN ENZYMATIC SYNTHESIS OF CATECHOLAMINES.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 228
2.3 hours for orally inhaled levodopa. Oral levodopa has a half life of 50 minutes but when combined with a peripheral dopa decarboxylase inhibitor, the half life is increased to 1.5 hours.
THE HALF-LIFE IN PLASMA IS SHORT (1-3 HR).
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 555
Levodopa by various routes crosses the blood brain barrier, is decarboxylated to form dopamine. This supplemental dopamine performs the role that endogenous dopamine cannot due to a decrease of natural concentrations and stimulates dopaminergic receptors.
MOST WIDELY ACCEPTED THEORY IS THAT LEVODOPA INCR LEVEL OF DOPAMINE & THUS ACTIVATION OF DOPAMINE RECEPTORS IN EXTRA-PYRAMIDAL CENTERS IN THE BRAIN (PRIMARILY IN CAUDATE NUCLEUS & SUBSTANTIA NIGRA).
Evaluations of Drug Interactions. 2nd ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1976, 1978., p. 124
The present data indicate that the major effects observed after administration of exogenous levodopa are not due to a direct action of levodopa on dopamine receptors, or to extrastriatal release of dopamine, but to conversion of levodopa to dopamine by serotonergic terminals and probably some intrastriatal cells.
PMID:11274784 Lopez A et al; Neuroscience 103(3) : 639-651 (2001)
EFFECTS OF LEVODOPA ON HUMAN & MURINE MELANOMA CELLS EXAMINED. WHEN EXPONENTIALLY GROWING CELLS WERE EXPOSED TO L-DOPA, CHARACTERISTIC INHIBITION OF THYMIDINE INCORPORATION OBSERVED.
PMID:6768447 WICK MM; CANCER RES 40 (5): 1414 (1980)
IN RATS, DOPAMINERGIC AGONISTS ALL CAUSED DECR IN SERUM PROLACTIN LEVELS.
HOROWSKI R ET AL; ARCH TOXICOL (SUPPL 2): 93 (1979)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
62
PharmaCompass offers a list of Etilevodopa API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Etilevodopa manufacturer or Etilevodopa supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Etilevodopa manufacturer or Etilevodopa supplier.
PharmaCompass also assists you with knowing the Etilevodopa API Price utilized in the formulation of products. Etilevodopa API Price is not always fixed or binding as the Etilevodopa Price is obtained through a variety of data sources. The Etilevodopa Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A CAS-59-92-7 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of CAS-59-92-7, including repackagers and relabelers. The FDA regulates CAS-59-92-7 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. CAS-59-92-7 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of CAS-59-92-7 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A CAS-59-92-7 supplier is an individual or a company that provides CAS-59-92-7 active pharmaceutical ingredient (API) or CAS-59-92-7 finished formulations upon request. The CAS-59-92-7 suppliers may include CAS-59-92-7 API manufacturers, exporters, distributors and traders.
click here to find a list of CAS-59-92-7 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A CAS-59-92-7 DMF (Drug Master File) is a document detailing the whole manufacturing process of CAS-59-92-7 active pharmaceutical ingredient (API) in detail. Different forms of CAS-59-92-7 DMFs exist exist since differing nations have different regulations, such as CAS-59-92-7 USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A CAS-59-92-7 DMF submitted to regulatory agencies in the US is known as a USDMF. CAS-59-92-7 USDMF includes data on CAS-59-92-7's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The CAS-59-92-7 USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of CAS-59-92-7 suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The CAS-59-92-7 Drug Master File in Japan (CAS-59-92-7 JDMF) empowers CAS-59-92-7 API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the CAS-59-92-7 JDMF during the approval evaluation for pharmaceutical products. At the time of CAS-59-92-7 JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of CAS-59-92-7 suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a CAS-59-92-7 Drug Master File in Korea (CAS-59-92-7 KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of CAS-59-92-7. The MFDS reviews the CAS-59-92-7 KDMF as part of the drug registration process and uses the information provided in the CAS-59-92-7 KDMF to evaluate the safety and efficacy of the drug.
After submitting a CAS-59-92-7 KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their CAS-59-92-7 API can apply through the Korea Drug Master File (KDMF).
click here to find a list of CAS-59-92-7 suppliers with KDMF on PharmaCompass.
A CAS-59-92-7 CEP of the European Pharmacopoeia monograph is often referred to as a CAS-59-92-7 Certificate of Suitability (COS). The purpose of a CAS-59-92-7 CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of CAS-59-92-7 EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of CAS-59-92-7 to their clients by showing that a CAS-59-92-7 CEP has been issued for it. The manufacturer submits a CAS-59-92-7 CEP (COS) as part of the market authorization procedure, and it takes on the role of a CAS-59-92-7 CEP holder for the record. Additionally, the data presented in the CAS-59-92-7 CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the CAS-59-92-7 DMF.
A CAS-59-92-7 CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. CAS-59-92-7 CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of CAS-59-92-7 suppliers with CEP (COS) on PharmaCompass.
A CAS-59-92-7 written confirmation (CAS-59-92-7 WC) is an official document issued by a regulatory agency to a CAS-59-92-7 manufacturer, verifying that the manufacturing facility of a CAS-59-92-7 active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting CAS-59-92-7 APIs or CAS-59-92-7 finished pharmaceutical products to another nation, regulatory agencies frequently require a CAS-59-92-7 WC (written confirmation) as part of the regulatory process.
click here to find a list of CAS-59-92-7 suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing CAS-59-92-7 as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for CAS-59-92-7 API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture CAS-59-92-7 as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain CAS-59-92-7 and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a CAS-59-92-7 NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of CAS-59-92-7 suppliers with NDC on PharmaCompass.
CAS-59-92-7 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of CAS-59-92-7 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right CAS-59-92-7 GMP manufacturer or CAS-59-92-7 GMP API supplier for your needs.
A CAS-59-92-7 CoA (Certificate of Analysis) is a formal document that attests to CAS-59-92-7's compliance with CAS-59-92-7 specifications and serves as a tool for batch-level quality control.
CAS-59-92-7 CoA mostly includes findings from lab analyses of a specific batch. For each CAS-59-92-7 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
CAS-59-92-7 may be tested according to a variety of international standards, such as European Pharmacopoeia (CAS-59-92-7 EP), CAS-59-92-7 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (CAS-59-92-7 USP).
