
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
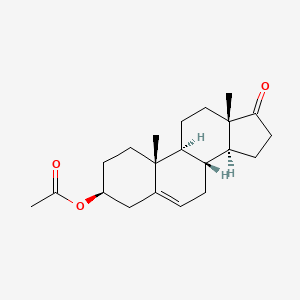

1. 853-23-6
2. Prasterone Acetate
3. Dehydroisoandrosterone 3-acetate
4. Androstenolone Acetate
5. Dehydroisoandrosterone Acetate
6. Dehydroepiandrosterone 3-acetate
7. Dhea Acetate
8. Trans-dehydroandrosterone Acetate
9. Androst-5-en-17-one, 3-(acetyloxy)-, (3b)-
10. 3beta-acetoxy-5-androstene-17-one
11. Dehydroisoandosterone 3-acetate
12. [(3s,8r,9s,10r,13s,14s)-10,13-dimethyl-17-oxo-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-3-yl] Acetate
13. 3beta-acetoxyandrost-5-en-17-one
14. 45751h45my
15. (3beta)-17-oxoandrost-5-en-3-yl Acetate
16. Dsstox_cid_380
17. Dsstox_rid_75554
18. Dsstox_gsid_20380
19. Ccris 7926
20. 3-beta-acetoxydehydroepiandrosterone
21. Skf 2847
22. Einecs 212-714-1
23. 5-androsten-3beta-ol-7-one Acetate
24. 17-oxoandrost-5-en-3beta-yl Acetate
25. Delta5-dehydroepiandrosterone 3-acetate
26. Dehydroepiandrost-5-en-17-one 3-acetate
27. 3beta-hydroxy-5-androsten-17-one Acetate
28. 3beta-hydroxyandrost-5-en-17-one Acetate
29. 3beta-hydroxy-5-androstene-17-one Acetate
30. 3-beta-hydroxyandrost-5-en-17-one Acetate
31. 3beta-hydroxyandrost-5-en-17-one 3-acetate
32. Unii-45751h45my
33. 17-oxoandrost-5-en-3-yl Acetate #
34. Prasterone-acetate
35. Ncgc00016544-01
36. Cas-853-23-6
37. Androst-5-en-17-one, 3-(acetyloxy)-, (3-beta)-
38. Prestwick0_000937
39. Prestwick1_000937
40. Prestwick2_000937
41. Prestwick3_000937
42. Androst-5-en-17-one, 3-beta-hydroxy-, Acetate
43. Bspbio_000874
44. Mls002154068
45. Schembl298142
46. Spbio_003043
47. Bpbio1_000962
48. Chembl1480686
49. Dtxsid3020380
50. Chebi:135410
51. 3betaacetoxy-5-androstene-17-one
52. Hms1570l16
53. Hms2097l16
54. Hms2230i06
55. Hms3714l16
56. 3beta-acetoxy-5-androsten-17-one
57. (3s,8r,9s,10r,13s,14s)-10,13-dimethyl-17-oxo-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl Acetate
58. Hy-b1405
59. Zinc3881408
60. 3beta-acetoxy-androst-5-en-17-one
61. Tox21_110485
62. Tox21_200963
63. S5508
64. 3-(acetyloxy)androst-5-ene-17-one
65. 3beta-acetoxy-5-androstene -17-one
66. Akos015895423
67. Ccg-220937
68. Cs-6082
69. Dehydroisoandrosterone 3-acetate, 97%
70. Fd12035
71. Ncgc00179388-01
72. Ncgc00179388-02
73. Ncgc00258516-01
74. As-20015
75. Smr001233383
76. (3?)-17-oxoandrost-5-en-3-yl Acetate
77. 5-androsten-3.beta.-ol-17-one, 3-acetate
78. Ab00171449
79. D0045
80. 853d236
81. Q-200934
82. Brd-k96527333-001-03-4
83. Q27258813
84. Acetic Acid (3s,8r,10r,13s)-10,13-dimethyl-17-oxo-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl Ester
| Molecular Weight | 330.5 g/mol |
|---|---|
| Molecular Formula | C21H30O3 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 330.21949481 g/mol |
| Monoisotopic Mass | 330.21949481 g/mol |
| Topological Polar Surface Area | 43.4 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 606 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Market Place

ABOUT THIS PAGE
91
PharmaCompass offers a list of Prasterone Acetate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Prasterone Acetate manufacturer or Prasterone Acetate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Prasterone Acetate manufacturer or Prasterone Acetate supplier.
PharmaCompass also assists you with knowing the Prasterone Acetate API Price utilized in the formulation of products. Prasterone Acetate API Price is not always fixed or binding as the Prasterone Acetate Price is obtained through a variety of data sources. The Prasterone Acetate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A CAS-853-23-6 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of CAS-853-23-6, including repackagers and relabelers. The FDA regulates CAS-853-23-6 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. CAS-853-23-6 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of CAS-853-23-6 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A CAS-853-23-6 supplier is an individual or a company that provides CAS-853-23-6 active pharmaceutical ingredient (API) or CAS-853-23-6 finished formulations upon request. The CAS-853-23-6 suppliers may include CAS-853-23-6 API manufacturers, exporters, distributors and traders.
click here to find a list of CAS-853-23-6 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A CAS-853-23-6 DMF (Drug Master File) is a document detailing the whole manufacturing process of CAS-853-23-6 active pharmaceutical ingredient (API) in detail. Different forms of CAS-853-23-6 DMFs exist exist since differing nations have different regulations, such as CAS-853-23-6 USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A CAS-853-23-6 DMF submitted to regulatory agencies in the US is known as a USDMF. CAS-853-23-6 USDMF includes data on CAS-853-23-6's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The CAS-853-23-6 USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of CAS-853-23-6 suppliers with USDMF on PharmaCompass.
CAS-853-23-6 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of CAS-853-23-6 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right CAS-853-23-6 GMP manufacturer or CAS-853-23-6 GMP API supplier for your needs.
A CAS-853-23-6 CoA (Certificate of Analysis) is a formal document that attests to CAS-853-23-6's compliance with CAS-853-23-6 specifications and serves as a tool for batch-level quality control.
CAS-853-23-6 CoA mostly includes findings from lab analyses of a specific batch. For each CAS-853-23-6 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
CAS-853-23-6 may be tested according to a variety of international standards, such as European Pharmacopoeia (CAS-853-23-6 EP), CAS-853-23-6 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (CAS-853-23-6 USP).
