
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
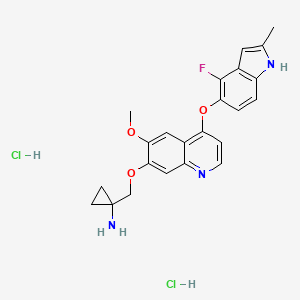

1. Anlotinib Hcl
2. 1360460-82-7
3. Anlotinib Hydrochloride
4. Al3818 Dihydrochloride
5. Catequentinib Hydrochloride
6. Catequentinib Dihydrochloride
7. A3749m6582
8. 1360460-82-7 (hcl)
9. 1-[[4-[(4-fluoro-2-methyl-1h-indol-5-yl)oxy]-6-methoxyquinolin-7-yl]oxymethyl]cyclopropan-1-amine;dihydrochloride
10. Catequentinib Hydrochloride (usan)
11. Anlotinib 2hcl
12. 1-(((4-((4-fluoro-2-methyl-1h-indol-5-yl)oxy)-6-methoxyquinolin-7-yl)oxy)methyl)cyclopropan-1-amine Dihydrochloride
13. 1-[({4-[(4-fluoro-2-methyl-1h-indol-5-yl)oxy]-6-methoxyquinolin-7-yl}oxy)methyl]cyclopropan-1-amine Dihydrochloride
14. Catequentinib Hydrochloride [usan]
15. 1-[[[4-[(4-fluoro-2-methyl-1h-indol-5-yl)oxy]-6-methoxy-7-quinolinyl]oxy]methyl]cyclopropanamine Hydrochloride (1:2); Al3818 Dihydrochloride
16. Chembl5314570
17. Schembl18691323
18. Al-3818 Dihydrochloride
19. Unii-a3749m6582
20. Bcp19682
21. Anlotinib (al3818) Dihydrochloride
22. Hy-19716a
23. S8726
24. Ccg-269545
25. Catequentinib Hydrochloride [who-dd]
26. Cs-0040093
27. D12537
28. D72434
29. 1-((4-((4-fluoro-2-methyl-1h-indol-5-yl)oxy)-6-methoxy-7-quinolyl)oxymethyl)cyclopropanamine, Dihydrochloride
30. 1-[[[4-(4-fluoro-2-methyl-1h-indol-5-yloxy)-6-methoxyquinolin-7-yl]oxy]methyl]cyclopropanamine Dihydrochloride
31. Cyclopropanamine, 1-(((4-((4-fluoro-2-methyl-1h-indol-5-yl)oxy)-6-methoxy-7-quinolinyl)oxy)methyl)-, Hydrochloride (1:2)
32. Cyclopropanamine, 1-[[[4-[(4-fluoro-2-methyl-1h-indol-5-yl)oxy]-6-methoxy-7-quinoliny]oxy]methyl]-, Hydrochloride (1:2)
1. 1058156-90-3
2. Anlotinib
| Molecular Weight | 480.4 g/mol |
|---|---|
| Molecular Formula | C23H24Cl2FN3O3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 82.4 |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 606 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
ABOUT THIS PAGE
