
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
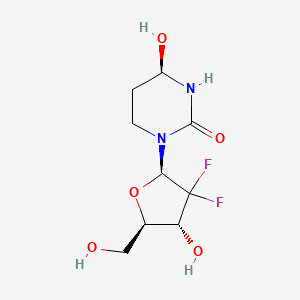

1. (4r)-2'-deoxy-2',2'-difluoro-3,4,5,6-tetrahydrouridine
1. 1141397-80-9
2. Cedazuridine [inn]
3. Cedazuridine [usan]
4. 39is23q1ew
5. Chembl3237547
6. E7727
7. Astx727 Component Cedazuridine
8. Astx-727 Component Cedazuridine
9. (4r)-2'-deoxy-2',2'-difluoro-3,4,5,6-tetrahydrouridine
10. E-7727
11. (4r)-1-[(2r,4r,5r)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-4-hydroxy-1,3-diazinan-2-one
12. Uridine, 2'-deoxy-2',2'-difluoro-3,4,5,6-tetrahydro-, (4r)-
13. (r)-1-((2r,4r,5r)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-4-hydroxytetrahydropyrimidin-2(1h)-one
14. Cedazuridine [usan:inn]
15. Unii-39is23q1ew
16. Cedazuridine [who-dd]
17. Schembl172256
18. Gtpl11101
19. Cedazuridine [orange Book]
20. Ex-a5549
21. Inqovi Component Cedazuridine
22. Bdbm50007029
23. Who 10741
24. Astx727 (cedazuridine + Decitabine)
25. At22227
26. Cedazuridine Component Of Inqovi
27. Compound 7a [pmid: 24520856]
28. A937507
29. (4r)-4-hydroxy-1-(2,2-difluoro-2-deoxy-beta-d-ribofuranosyl)-3,4,5,6-tetrahydropyrimidine-2(1h)-one
| Molecular Weight | 268.21 g/mol |
|---|---|
| Molecular Formula | C9H14F2N2O5 |
| XLogP3 | -1.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 268.08707788 g/mol |
| Monoisotopic Mass | 268.08707788 g/mol |
| Topological Polar Surface Area | 102 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 343 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cedazuridine, in combination with decitabine, is indicated for the treatment of myelodysplastic syndromes (MDS), including MDS with refractory anemia, MDS with refractory anemia and ringed sideroblasts, MDS with refractory anemia and excess blasts, MDS scoring intermediate-1, intermediate-2, or high-risk on the International Prognostic Scoring System (IPSS), and chronic myelomonocytic leukemia (CMML).
Cedazuridine is a cytidine deaminase inhibitor that is co-administered with hypomethylating agents such as [decitabine] in order to increase their oral bioavailability. In combination with hypomethylating agents, cedazuridine may cause myelosuppression and embryo-fetal toxicity and should be administered with appropriate monitoring.
Absorption
Cedazuridine (100 mg) taken orally with [decitabine] (35 mg) once daily for five days resulted in a day 1 AUC and steady-state AUC (coefficient of variation) of 103 (55%) and 178 (53%) ng\*hr/mL for [decitabine] and 2950 (49%) and 3291 (45%) ng\*hr/mL for cedazuridine, respectively. Overall, the 5-day cumulative AUC for [decitabine] was 851 (50%). Similarly, the Cmax for [decitabine] and cedazuridine was 145 (55%) and 371 (52%) ng/mL, respectively. The median Tmax for [decitabine] was 1 hr (range 0.3 to 3.0 hrs) and for cedazuridine was 3 hrs (range 1.5 to 6.1 hrs). The bioavailability of [decitabine], as assessed by comparing the AUC of oral [decitabine] co-administered with cedazuridine to intravenous [decitabine] alone, was 60% on day 1 (90% CI of 55-65%). The corresponding values on day 5 and considering the cumulative day 5 dose were 106% (90% CI: 98, 114) and 99% (90% CI: 93, 106). Hence, the oral bioavailability of [decitabine] approaches 100% over the 5-day treatment cycle.
Route of Elimination
Roughly 46% of cedazuridine is found in urine, 21% of which is unchanged, and 51% is found in feces, 27% of which is unchanged.
Volume of Distribution
The apparent volume of distribution (and coefficient of variation) of [decitabine] and cedazuridine at steady state was 417 (54%) and 296 (51%), respectively.
Clearance
Cedazuridine has an apparent steady-state clearance of 30.3 L/hours, with a coefficient of variation of 46%.
The metabolism of cedazuridine is not well-established. Cedazuridine is known to be converted to an epimer that is roughly 10-fold less effective in inhibiting cytidine deaminase and is subsequently degraded through unknown pathways.
Cedazuridine has a steady-state half-life of 6.7 hours, with a coefficient of variation of 19%.
Myelodysplastic syndromes (MDS) represent a heterogeneous group of hematopoietic neoplasms arising from a variety of underlying mutations that manifest in peripheral cytopenias and may eventually progress to secondary acute myeloid leukemia (sAML). There are over 45 genes commonly mutated in MDS patients, including those involved in DNA methylation and repair, histone modification, RNA splicing, transcription, signal transduction, and cellular adhesion. It is hypothesized that initial clonal founder mutations give rise to progressive acquisition of secondary mutations and facilitate disease progression to sAML. Hypomethylating agents such as [decitabine] are metabolized into triphosphate derivatives that are subsequently incorporated into DNA. Once incorporated, these agents inhibit the activity of DNA methylases such as DNMT1, leading to progressive DNA hypomethylation and eventual activation of tumour suppression genes and apoptotic pathways. However, hypomethylating agents given orally are vulnerable to first-pass metabolism by cytidine deaminase, and hence typically have to be administered through intramuscular or intravenous routes. Co-administration with cedazuridine, which is an efficient inhibitor of cytidine deaminase, drastically increases the oral bioavailability of [decitabine], allowing for combination oral therapy.
Global Sales Information

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
17
PharmaCompass offers a list of Cedazuridine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Cedazuridine manufacturer or Cedazuridine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Cedazuridine manufacturer or Cedazuridine supplier.
PharmaCompass also assists you with knowing the Cedazuridine API Price utilized in the formulation of products. Cedazuridine API Price is not always fixed or binding as the Cedazuridine Price is obtained through a variety of data sources. The Cedazuridine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Cedazuridine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Cedazuridine, including repackagers and relabelers. The FDA regulates Cedazuridine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Cedazuridine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Cedazuridine supplier is an individual or a company that provides Cedazuridine active pharmaceutical ingredient (API) or Cedazuridine finished formulations upon request. The Cedazuridine suppliers may include Cedazuridine API manufacturers, exporters, distributors and traders.
click here to find a list of Cedazuridine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Cedazuridine DMF (Drug Master File) is a document detailing the whole manufacturing process of Cedazuridine active pharmaceutical ingredient (API) in detail. Different forms of Cedazuridine DMFs exist exist since differing nations have different regulations, such as Cedazuridine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Cedazuridine DMF submitted to regulatory agencies in the US is known as a USDMF. Cedazuridine USDMF includes data on Cedazuridine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Cedazuridine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Cedazuridine suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Cedazuridine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Cedazuridine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Cedazuridine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Cedazuridine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Cedazuridine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Cedazuridine suppliers with NDC on PharmaCompass.
Cedazuridine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Cedazuridine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Cedazuridine GMP manufacturer or Cedazuridine GMP API supplier for your needs.
A Cedazuridine CoA (Certificate of Analysis) is a formal document that attests to Cedazuridine's compliance with Cedazuridine specifications and serves as a tool for batch-level quality control.
Cedazuridine CoA mostly includes findings from lab analyses of a specific batch. For each Cedazuridine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Cedazuridine may be tested according to a variety of international standards, such as European Pharmacopoeia (Cedazuridine EP), Cedazuridine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Cedazuridine USP).
