
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
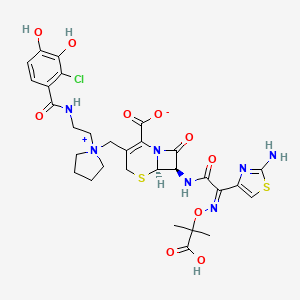

1. S-649266
1. 1225208-94-5
2. Cefiderocol [inn]
3. Gsk2696266
4. Cefiderocol [who-dd]
5. Sz34omg6e8
6. S-649266
7. Cefiderocol (usan)
8. Cefiderocol [usan]
9. (6r,7r)-7-[[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(2-carboxypropan-2-yloxyimino)acetyl]amino]-3-[[1-[2-[(2-chloro-3,4-dihydroxybenzoyl)amino]ethyl]pyrrolidin-1-ium-1-yl]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
10. Pyrrolidinium, 1-(((6r,7r)-7-(((2z)-2-(2-amino-4-thiazolyl)-2-((1-carboxy-1-methylethoxy)imino)acetyl)amino)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-1-(2-((2-chloro-3,4-dihydroxybenzoyl)amino)ethyl)-, Inner Salt
11. Rsc 649266
12. Pyrrolidinium, 1-[[(6r,7r)-7-[[(2z)-2-(2-amino-4-thiazolyl)-2-[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-[2-[(2-chloro-3,4-dihydroxybenzoyl)amino]ethyl]-, Inner Salt
13. Cefiderocol [mi]
14. Cefiderocol [usan:inn]
15. Unii-sz34omg6e8
16. Chembl3989974
17. Schembl22508010
18. Dtxsid401098052
19. Akos037648584
20. Db14879
21. Gsk 2696266
22. Bs-14716
23. Hy-17628
24. Cs-0016784
25. D11302
26. S 649266
27. (6r,7r)-7-((2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(((2-carboxypropan-2-yl)oxy)imino)acetamido)-3-((1-(2-(2-chloro-3,4-dihydroxybenzamido)ethyl)pyrrolidin-1-ium-1-yl)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylate
| Molecular Weight | 752.2 g/mol |
|---|---|
| Molecular Formula | C30H34ClN7O10S2 |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 12 |
| Exact Mass | 751.1497103 g/mol |
| Monoisotopic Mass | 751.1497103 g/mol |
| Topological Polar Surface Area | 310 Ų |
| Heavy Atom Count | 50 |
| Formal Charge | 0 |
| Complexity | 1440 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cefiderocol is indicated for the treatment of complicated urinary tract infections with or without pyelonephritis.
FDA Label
Fetcroja is indicated for the treatment of infections due to aerobic Gram-negative organisms in adults with limited treatment options (see sections 4. 2, 4. 4 and 5. 1).
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Treatment of infections due to aerobic Gram-negative bacteria
Similarly to other cephalosporins, cefiderocol exerts bactericidal activity against a range of bacterial species. Cefiderocol has primarily shown efficacy against aerobic Gram negative bacteria including *Escherichia coli*, *Klebsiella pneumoniae*, and *Pseudomonas aeruginosa*.
J01D
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DI - Other cephalosporins and penems
J01DI04 - Cefiderocol
Absorption
A single intravenous dose of 2 g of cefiderocol in healthy patients produces a Cmax of 89.7 mg/L and an AUC of 386 mg\*h/L. In patients with complicated urinary tract infections and a creatinine clearance of at least 60 mL/min, doses of 2 g cefiderocol every 8 hours produced an AUC of 394.7 mg*h/L and a Cmax of 138 mg/L. However the infusion rate for this chronic dosing was 3 times the recommended rate. Cmax and AUC are known to increase proportionally with dosage.
Route of Elimination
98.6% of cefiderocol is eliminated in the urine with 90.6% as the unchanged parent drug. The remaining 8% is eliminated as metabolites. 2.8% is eliminated in the feces. Less than 10% of cefiderocol is metabolized.
Volume of Distribution
Cefiderocol has a mean volume of distribution of 18 L.
Clearance
Cefiderocol has a mean clearance of 5.18 L/h.
Cefiderocol undergoes a small degree of metabolism to a cefiderocol epimer at the 7 position, cefiderocol catechol-3-methoxy and -4-methoxy, and a pyrrolidine chlorobenzamide product (PCBA). PCBA undergoes further metabolism to sulfated, methylated, and glucuronidated metabolites. The enzymes involved in these reactions have yet to be identified and cefiderocol has not been shown to interfere in the metabolism of other agents.
The terminal elimination half-life of cefiderocol is 2-3 h.
Cefiderocol acts by binding to and inhibiting penicillin-binding proteins (PBPs), preventing cell wall synthesis and ultimately causing death of the bacterial cell. Like other -lactam antibiotics cefiderocol is able to enter bacterial cells via passive diffusion through porins. Unlike other -lactams, cefiderocol contains a chlorocatechol group which allows it to chelate iron. Once bound to ferric iron cefiderocol is able to undergo active transport into bacterial cells through iron channels in the outer cell membrane such as those encoded by the *cirA* and *fiu* genes in *E. coli* or the *PiuA* gene in *P. aeruginosa*. Once inside the cell, cefiderocol binds to and inhibits PBP3 with high affinity thereby preventing the linking of peptodoglycan layers via the pentapeptide bridge. PBP1a, 1b, 2,and 4 are also bound and inhibited by cefiderocol but with a lesser potency than PBP3 and are therefore expected to contribute less to its antibacterial effect.
 Jinan Tantu Chemicals offers customized R&D services & production of small molecule APIs & pharmaceutical intermediates.
Jinan Tantu Chemicals offers customized R&D services & production of small molecule APIs & pharmaceutical intermediates.
About the Company : Jinan Tantu Chemicals Co., Ltd. operates as a Contract Development and Manufacturing Organization (CDMO) that serves pharmaceutical companies worldwide. Our core services include c...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
2-chloro-3,4-bis((4-methoxybenzyl)oxy)-N-(2-(pyrro...
CAS Number : CAS-1225208-44-5
End Use API : Cefiderocol
About The Company : Jinan Tantu Chemicals Co., Ltd. operates as a Contract Development and Manufacturing Organization (CDMO) that serves pharmaceutical companies worldwide. Our cor...
2-chloro-3,4-bis[(4-methoxyphenyl)methoxy]-Benzald...
CAS Number : CAS-1884263-19-7
End Use API : Cefiderocol
About The Company : Jinan Tantu Chemicals Co., Ltd. operates as a Contract Development and Manufacturing Organization (CDMO) that serves pharmaceutical companies worldwide. Our cor...
7-Amino-3-chloromethyl-3-cephem-4-carboxylic acid ...
CAS Number : CAS-113479-65-5
End Use API : Cefiderocol
About The Company : Jinan Tantu Chemicals Co., Ltd. operates as a Contract Development and Manufacturing Organization (CDMO) that serves pharmaceutical companies worldwide. Our cor...
2-chloro-3,4-bis(4-methoxybenzyloxy)benzoic acid
CAS Number : CAS-137054-46-7
End Use API : Cefiderocol
About The Company : Jinan Tantu Chemicals Co., Ltd. operates as a Contract Development and Manufacturing Organization (CDMO) that serves pharmaceutical companies worldwide. Our cor...
2-(((1-(tert-Butoxy)-2-methyl-1-oxopropan-2-yl)oxy...
CAS Number : 137088-65-4
End Use API : Cefiderocol
About The Company : Jinan Tantu Chemicals Co., Ltd. operates as a Contract Development and Manufacturing Organization (CDMO) that serves pharmaceutical companies worldwide. Our cor...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

ABOUT THIS PAGE
53
PharmaCompass offers a list of Cefiderocol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Cefiderocol manufacturer or Cefiderocol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Cefiderocol manufacturer or Cefiderocol supplier.
PharmaCompass also assists you with knowing the Cefiderocol API Price utilized in the formulation of products. Cefiderocol API Price is not always fixed or binding as the Cefiderocol Price is obtained through a variety of data sources. The Cefiderocol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Cefiderocol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Cefiderocol, including repackagers and relabelers. The FDA regulates Cefiderocol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Cefiderocol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Cefiderocol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Cefiderocol supplier is an individual or a company that provides Cefiderocol active pharmaceutical ingredient (API) or Cefiderocol finished formulations upon request. The Cefiderocol suppliers may include Cefiderocol API manufacturers, exporters, distributors and traders.
click here to find a list of Cefiderocol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Cefiderocol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Cefiderocol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Cefiderocol GMP manufacturer or Cefiderocol GMP API supplier for your needs.
A Cefiderocol CoA (Certificate of Analysis) is a formal document that attests to Cefiderocol's compliance with Cefiderocol specifications and serves as a tool for batch-level quality control.
Cefiderocol CoA mostly includes findings from lab analyses of a specific batch. For each Cefiderocol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Cefiderocol may be tested according to a variety of international standards, such as European Pharmacopoeia (Cefiderocol EP), Cefiderocol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Cefiderocol USP).
