
Synopsis
Synopsis
0
JDMF
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
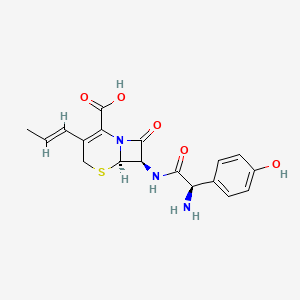

1. 7-(2-amino-2-(4-hydroxyphenyl)acetamido)-3-(propenyl)-3-cephem-4-carboxylic Acid Monohydrate
2. 7-(2-amino-2-(p-hydroxyphenyl)acetamido)-8-oxo-3-(1-propenyl)-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
3. Arzimol
4. Bmy 28100
5. Bmy-28100
6. Brisoral
7. Cefprozil Monohydrate
8. Cefzil
9. Procef
1. Cefprozil Anhydrous
2. Cefzil
3. Cefprozilum
4. 92665-29-7
5. Cefprozilo
6. Brisoral
7. Cefprozilum [inn-latin]
8. Cefprozilo [inn-spanish]
9. Procef
10. Trans-cefprozil
11. 92676-86-3
12. Arzimol
13. S1sdi2fjiy
14. Cronocef
15. Serozil
16. Cefzil (tn)
17. Cefprozil Anhydrous, E-isomer
18. Bmy 28100
19. Cefprozil Hydrate (cefzil)
20. Cefprozil (e)-isomer (50 Mg)g0d341872ug/mg(ai)
21. (6s,7r)-7-((r)-2-amino-2-(4-hydroxyphenyl)acetamido)-8-oxo-3-((e)-prop-1-en-1-yl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
22. Cefprozil (e)-isomer
23. Cefprozil (inn)
24. Cefprozil (tn)
25. Cefprozil [usan:inn]
26. Chebi:3506
27. Unii-1m698f4h4e
28. E-cefprozil
29. (e)-cefprozil
30. Bbs-1067
31. Bmy-28167
32. Unii-s1sdi2fjiy
33. Cefprozil [mart.]
34. Schembl37024
35. Bidd:gt0833
36. Cefprozil E-form [mi]
37. Chembl276568
38. Cefprozil For Peak Identification
39. Hy-b0458a
40. Dtxsid10873545
41. N,n-bisbenzylidenebenzidine
42. 1m698f4h4e
43. Hms3715p22
44. Cefprozil Anhydrous, (e)-
45. Zinc3776970
46. Akos015895989
47. Ccg-221280
48. Db01150
49. (6r,7r)-7-{[(2r)-2-amino-2-(4-hydroxyphenyl)acetyl]amino}-8-oxo-3-(prop-1-en-1-yl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
50. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2r)-amino(4-hydroxyphenyl)acetyl)amino)-8-oxo-3-(1-propenyl)-, (6r,7r)-
51. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((amino(4-hydroxyphenyl)acetyl)amino)-8-oxo-3-(1-propenyl)-, (6r-(6alpha,7beta(r*)))-
52. As-14301
53. Discontinued. Please See C243933.
54. Cs-0013517
55. C06888
56. C16732
57. D07651
58. Ab01274812-01
59. Ab01274812_02
60. 665c297
61. Cefprozil, Mix Of Z (92%), And E (7%) Isomers
62. Q3231623
63. (6r,7r)-7-((r)-2-amino-2-(4-hydroxyphenyl)acetamido)-8-oxo-3-((e)-prop-1-en-1-yl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
64. (6r,7r)-7-((r)-2-amino-2-(4-hydroxyphenyl)acetamido)-8-oxo-3-((e)-prop-1-en-1-yl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylicacid
65. (6r,7r)-7-((r)-2-amino-2-(4-hydroxyphenyl)acetamido)-8-oxo-3-((e)-prop-1-enyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
66. (6r,7r)-7-((r)-2-amino-2-(p-hydroxyphenyl)acetamido)-8-oxo-3-propenyl-5-thia-1-azabicyclo(4.2.0)oct-2(e)-ene-2-carboxylic Acid
67. (6r,7r)-7-[(2r)-2-amino-2-(4-hydroxyphenyl)acetamido]-8-oxo-3-(prop-1-en-1-yl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
68. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2r)-amino(4-hydroxyphenyl)acetyl)amino)-8-oxo-3-(1e)-1-propenyl-,(6r,7r)-
| Molecular Weight | 389.4 g/mol |
|---|---|
| Molecular Formula | C18H19N3O5S |
| XLogP3 | -1.4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 389.10454189 g/mol |
| Monoisotopic Mass | 389.10454189 g/mol |
| Topological Polar Surface Area | 158 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 699 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Cefprozil |
| PubMed Health | Cefprozil (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Cefprozil is a semi-synthetic broad-spectrum cephalosporin antibiotic.Cefprozil is a cis and trans isomeric mixture (90% cis). The chemical name for the monohydrate is (6R,7R)-7-[(R)-2-amino-2-(p-hydroxyphenyl)acetamido]-8-oxo-3-propenyl-5-thia-1-... |
| Active Ingredient | Cefprozil |
| Dosage Form | Tablet; For suspension |
| Route | Oral |
| Strength | 250mg; 125mg/5ml; 500mg; 250mg/5ml |
| Market Status | Prescription |
| Company | Wockhardt; Teva Pharms; Teva; Apotex; Aurobindo Pharma; Lupin; Sandoz; Orchid Hlthcare |
| 2 of 2 | |
|---|---|
| Drug Name | Cefprozil |
| PubMed Health | Cefprozil (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Cefprozil is a semi-synthetic broad-spectrum cephalosporin antibiotic.Cefprozil is a cis and trans isomeric mixture (90% cis). The chemical name for the monohydrate is (6R,7R)-7-[(R)-2-amino-2-(p-hydroxyphenyl)acetamido]-8-oxo-3-propenyl-5-thia-1-... |
| Active Ingredient | Cefprozil |
| Dosage Form | Tablet; For suspension |
| Route | Oral |
| Strength | 250mg; 125mg/5ml; 500mg; 250mg/5ml |
| Market Status | Prescription |
| Company | Wockhardt; Teva Pharms; Teva; Apotex; Aurobindo Pharma; Lupin; Sandoz; Orchid Hlthcare |
For the treatment of the following infections (respiratory, skin, soft tissue, UTI, ENT) caused by; S. pneumoniae, H. influenzae, staphylococci, S. pyogenes (group A beta-hemolytic streptococci), E. coli, P. mirabilis, Klebsiella sp, coagulase-negative staph
FDA Label
Cefprozil, a semisynthetic, second-generation cephalosporin, is used to treat otitis media, soft-tissue infections, and respiratory tract infections.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DC - Second-generation cephalosporins
J01DC10 - Cefprozil
Absorption
Oral bioavailability is approximately 95%.
Volume of Distribution
0.23 L/kg
Clearance
3 mL/min/kg [fasting subjects]
Cefprozil is eliminated primarily by the kidneys
1.3 hours
Cefprozil, like the penicillins, is a beta-lactam antibiotic. By binding to specific penicillin-binding proteins (PBPs) located inside the bacterial cell wall, it inhibits the third and last stage of bacterial cell wall synthesis. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins; it is possible that cefprozil interferes with an autolysin inhibitor.
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?