
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
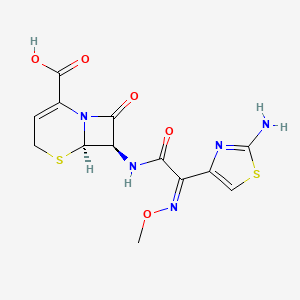

1. Cefizox
2. Ceftizoxime Monosodium Salt
3. Ceftizoxime Sodium
4. Fk 749
5. Fk-749
6. Fk749
7. Fr 13749
8. Fr-13749
9. Fr13749
10. Monosodium Salt, Ceftizoxime
11. Salt, Ceftizoxime Monosodium
12. Sk And F 88373 2
13. Sk And F 88373-2
14. Sk And F 883732
15. Skf 88373
16. Skf-88373
17. Skf88373
18. Sodium, Ceftizoxime
1. 68401-81-0
2. Ceftizoxima
3. Ceftizoximum
4. Cefizox
5. Ceftizoximum [inn-latin]
6. Ceftizoxima [inn-spanish]
7. Epocelin
8. Fk-749
9. Ceftizoxime (inn)
10. Syn-7-(2-(2-amino-4-thiazolyl)-2-methoxyiminoacetamido)-3-cephem-4-carboxylic Acid
11. C43c467dpe
12. (6r,7r)-7-[[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
13. Chebi:553473
14. Ceftix
15. Ceftizoxime [inn]
16. Ceftizoxime [inn:ban]
17. (6r,7r)-7-[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
18. Ceftisomin
19. Unii-c43c467dpe
20. (6r,7r)-7-(((2z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl)amino)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
21. Mfcd00072000
22. (6r,7r)-7-(2-(2-amino-4-thiazolyl)-2z-(methoxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-2-carbonsaeure
23. (6r,7r)-7-(2-(2-amino-4-thiazolyl)glyoxyamido)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-carbonsaeure-7-(z)-(o-methyloxim)
24. Fr-13479
25. Ceftizoxime [mi]
26. Czx
27. Ceftizoxime [jan]
28. Chembl528
29. Ceftizoxime [vandf]
30. Schembl37504
31. Ceftizoxime [usp-rs]
32. Ceftizoxime [who-dd]
33. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-8-oxo-, (6r-(6alpha,7beta(z)))-
34. Dtxsid5022772
35. Bdbm237182
36. (6r,7r)-7-((z)-2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
37. Hy-b1596
38. Zinc3871970
39. S4812
40. Akos025310621
41. Ccg-268439
42. Db01332
43. (6r,7r)-7-({(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(methyloxy)imino]acetyl}amino)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
44. (6r,7r)-7-{[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
45. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2-amino-4- Thiazolyl)(methoxyimino)acetyl)amino)-8-oxo-, (6r-(6-alpha,7-beta(z)))-
46. 7beta-{[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-2,3-didehydropenam-2-carboxylic Acid
47. Cs-0013519
48. C06890
49. D07658
50. 401c810
51. A836131
52. (6r,7r)-7-[[(2z)-2-(2-amino-4-thiazolyl)-2-methoxyimino-1-oxoethyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
53. (6r,7r)-7-[[(2z)-2-(2-azanyl-1,3-thiazol-4-yl)-2-methoxyimino-ethanoyl]amino]-8-oxidanylidene-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
54. (6r,7r)-7-[[(2z)-2-(2-imino-3h-thiazol-4-yl)-2-methoxyimino-acetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
55. 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid, 7-[[(2z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-, (6r,7r)-
| Molecular Weight | 383.4 g/mol |
|---|---|
| Molecular Formula | C13H13N5O5S2 |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 5 |
| Exact Mass | 383.03581088 g/mol |
| Monoisotopic Mass | 383.03581088 g/mol |
| Topological Polar Surface Area | 201 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 669 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cetizoxime was previously indicated for the treatment of infections due to susceptible strains of bacteria.
Ceftizoxime is highly resistant to a broad spectrum of beta-lactamases and is active against a wide range of both aerobic and anaerobic gram-positive and gram-negative organisms. It has few side effects and is reported to be safe and effective in aged patients and in patients with hematologic disorders.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DD - Third-generation cephalosporins
J01DD07 - Ceftizoxime
Route of Elimination
Excreted virtually unchanged by the kidneys in 24 hours.
Volume of Distribution
The mean apparent volume of distribution ranges between 15 - 28L.
Ceftizoxime is not metabolized and is excreted virtually unchanged by the kidneys in 24 hours.
Ceftizoxime is an aminothiazolyl cephalosporin with an extended spectrum of activity against many gram-negative, nosocomially acquired pathogens. It has excellent beta-lactamase stability, with good in vitro activity against Haemophilus influenzae, Neisseria gonorrhoeae and Klebsiella pneumoniae. Ceftizoxime, like the penicillins, is a beta-lactam antibiotic. By binding to specific penicillin-binding proteins (PBPs) located inside the bacterial cell wall, it inhibits the third and last stage of bacterial cell wall synthesis. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins; it is possible that ceftizoxime interferes with an autolysin inhibitor.
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
