

Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
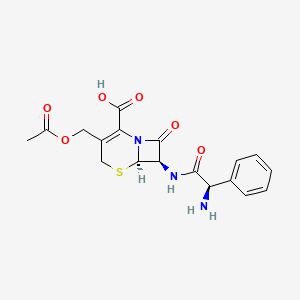

1. Cefaloglycin
2. Cephaloglycin Dihydrate
3. Cephaloglycine
4. Dihydrate, Cephaloglycin
1. Cefaloglycin
2. Cephaloglycine
3. D-cephaloglycine
4. Cephaoglycin Acid
5. Cephaloglycin Anhydrous
6. 3577-01-3
7. Cefaloglycine
8. Cefaloglicina
9. Cefaloglycinum
10. D-(-)-cephaloglycin
11. 7-(d-alpha-aminophenyl-acetamido)cephalosporanic Acid
12. 7-(2-d-alpha-aminophenylacetamido)cephalosporanic Acid
13. Cefaloglycin (jan)
14. Cefaloglycin [inn]
15. Chebi:34613
16. Kefglycin
17. (6r,7r)-3-(acetyloxymethyl)-7-[[(2r)-2-amino-2-phenylacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
18. Ceg
19. Cephaloglycin Dihydrate
20. Hd2d469w6u
21. Kefocin
22. 7-(d-2-amino-2-phenylacetamido)-3-acetoxymethyl-delta(sup3)-cephem-4-carboxylic Acid
23. Cefaloglycin [jan]
24. Cefaloglycin Dihydrate
25. Cefaloglycine [inn-french]
26. Cefaloglycinum [inn-latin]
27. Cefaloglicina [inn-spanish]
28. Lilly 39435
29. 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid,3-[(acetyloxy)methyl]-7-[[(2r)-aminophenylacetyl]amino]-8-oxo-,(6r,7r)-
30. Hsdb 3214
31. Einecs 222-696-7
32. Cephaloglycin Anhdyous
33. 7-(2-amino-2-phenylacetamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid Acetate (ester)
34. Cephaloglycin [mi]
35. Epitope Id:174844
36. Schembl2947
37. 7-(d-2-amino-2-phenylacetamido)-3-acetoxymethyl-delta(sup 3)-cephem-4-carboxylic Acid
38. Cephaloglycin [hsdb]
39. Unii-hd2d469w6u
40. 3-((acetyloxy)methyl)-7-((aminophenylacetyl)amino)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
41. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 3-((acetyloxy)methyl)-7-((aminophenylacetyl)amino)-8-oxo-, (6r-(6alpha,7beta(r*)))-
42. 7-(2-amino-2-phenylacetamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)octane-2-carboxylic Acid, Acetate Inner Salt
43. Cefaloglycin [who-dd]
44. Chembl1200971
45. Dtxsid4022781
46. Gtpl12193
47. Zinc3830503
48. Db00689
49. 3-acetoxymethyl-7beta-[(2r)-2-amino-2-phenylacetamido]-3,4-didehydrocepham-4-carboxylic Acid
50. Ncgc00521078-02
51. (6r,7r)-3-(acetoxymethyl)-7-{[(2r)-2-amino-2-phenylacetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
52. (6r,7r)-3-[(acetyloxy)methyl]-7-{[(2r)-2-amino-2-phenylacetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
53. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(2-amino-2-phenylacetamido)-3-(hydroxymethyl)-8-oxo-, Acetate (ester), D-
54. Hy-16137
55. Cs-0006156
56. D01949
57. Q5057214
58. (6r,7r)-3-[(acetyloxy)methyl]-7-[(2r)-2-amino-2-phenylacetamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
59. (6r,7r)-7-((r)-2-amino-2-phenylacetamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid Acetate (ester)
60. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-carboxylic Acid, 3-((acetyloxy)methyl)-7-((aminophenylacetyl)amino)-8-oxo-, (6r-(6.alpha.,7.beta.(r*)))
| Molecular Weight | 405.4 g/mol |
|---|---|
| Molecular Formula | C18H19N3O6S |
| XLogP3 | -3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 7 |
| Exact Mass | 405.09945651 g/mol |
| Monoisotopic Mass | 405.09945651 g/mol |
| Topological Polar Surface Area | 164 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 718 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cephalosporins
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
...BECAUSE CEPHALOGLYCIN IS CONCENTRATED IN URINE, IT MAY BE EFFECTIVE IN TREATMENT OF URINARY TRACT INFECTIONS... /DIHYDRATE/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1120
MOST OF CEPHALOGLYCIN...IS EXCRETED IN URINE AS DEACETYLCEPHALOGLYCIN, AN ANTIMICROBIALLY ACTIVE METABOLITE. URINARY CONCN OF METABOLITE AVG 350 UG/ML OVER 8-HR PERIOD FOLLOWING ADMIN OF 500 MG OF CEPHALOGLYCIN; THIS IS SUFFICIENT TO INHIBIT MOST STRAINS OF E COLI, MIRABILIS, & KLEBSIELLA-ENTEROBACTER (AEROBACTER) GROUP.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1162
ITS IN VITRO SPECTRUM IS SIMILAR TO THAT OF OTHER CEPHALOSPORINS...& INCL MANY PATHOGENS THAT INFECT URINARY TRACT (EG, ESCHERICHIA COLI, CERTAIN SPECIES OF KLEBSIELLA, STAPHYLOCOCCUS AUREUS). /DIHYDRATE/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 722
For more Therapeutic Uses (Complete) data for CEPHALOGLYCIN (7 total), please visit the HSDB record page.
...CEPHALOGLYCIN IS NOT DRUG OF CHOICE FOR TREATING URINARY TRACT INFECTIONS... IS NOT EVEN A CEPHALOSPORIN OF 1ST CHOICE FOR SEVERE URINARY TRACT INFECTIONS. AVAILABILITY OF...CEPHALOSPORINS...EXCRETED BY KIDNEY...ACHIEVING HIGHER BACTERICIDAL CONCN IN URINE THAN CEPHALOGLYCIN...RENDERED CEPHALOGLYCIN OBSOLETE. /DIHYDRATE/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 722
CEPHALOGLYCIN IS INEFFECTIVE AGAINST PSEUDOMONAS, & MOST SPECIES OF ENTEROCOCCI, ENTEROBACTER, & INDOLE-POSITIVE PROTENS. BECAUSE OF LIMITED ABSORPTION, SERUM LEVELS OF DRUG ADEQUATE TO TREAT SYSTEMIC INFECTIONS CANNOT BE ATTAINED CLINICALLY. /DIHYDRATE/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 722
SUPRAINFECTIONS, USUALLY DUE TO GRAM-NEGATIVE BACTERIA, MAY OCCUR WHEN THESE ANTIBIOTICS ARE EMPLOYED. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1163
Hypersensitivity reactions to the cephalosporins are the most common side effects...the reactions appear to be identical to those caused by the penicillins. /Cephalosporins/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1095
For more Drug Warnings (Complete) data for CEPHALOGLYCIN (12 total), please visit the HSDB record page.
For treatment of severe infections caused by susceptible bacteria.
Cephaloglycin is an antibiotic related to cephalosporin but no longer in common use. It is an orally absorbed derivative of cephalosporin C.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Absorption
Well absorbed following oral administration.
MOST OF CEPHALOGLYCIN THAT IS ABSORBED IS EXCRETED IN URINE AS DEACETYLCEPHALOGLYCIN, AN ANTIMICROBIALLY ACTIVE METABOLITE. URINARY CONCN OF METABOLITE AVG 350 UG/ML OVER 8-HR PERIOD FOLLOWING ADMIN OF 500 MG OF CEPHALOGLYCIN...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1162
CEPHALOGLYCIN IS NOT DESTROYED IN GASTRIC ACID, BUT IT GRADUALLY DECOMP IN NEUTRAL & ALKALINE SECRETIONS OF INTESTINES. ONLY 1/4 TO 1/3 IS ABSORBED. /DIHYDRATE/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1120
...CEPHALOSPORINS ARE UNPREDICTABLE IN MANNER IN WHICH THEY CROSS BLOOD-BRAIN BARRIER & THEY PENETRATE POORLY INTO CEREBROSPINAL FLUID... /CEPHALOSPORINS/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 713
MOST OF CEPHALOGLYCIN THAT IS ABSORBED IS EXCRETED...AS DEACETYLCEPHALOGLYCIN, AN ANTIMICROBIALLY ACTIVE METABOLITE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1162
THIS ANTIBIOTIC IS PARTIALLY ABSORBED FROM GI TRACT... PLASMA CONCN REACH PEAK OF ONLY 2-6 UG/ML @ ABOUT 2 HR FOLLOWING SINGLE DOSE OF 0.5 G; DRUG IS NO LONGER DETECTABLE IN PLASMA 8 HR AFTER THIS DOSE. HALF-LIFE...IS ABOUT 4 HR.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1162
PLASMA LEVELS AFTER ORAL ADMIN VARY WIDELY AMONG PT. PEAK LEVEL OF AT LEAST 0.6 UG/ML IS USUALLY ACHIEVED WITH ORAL DOSE OF 500 MG, & IT MAY REACH 6 UG/ML IN FAIR PROP OF CASES. FOOD IN STOMACH INTERFERES WITH ABSORPTION. HALF-LIFE IN PLASMA...ABOUT 3-5 HR, BUT WHEN...SEVERE RENAL IMPAIRMENT...MAY BE 9-20 HR. /DIHYDRATE/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1120
The bactericidal activity of cephaloglycin results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins (PBPs).
CEPHALOTHIN & ITS CONGENERS INHIBIT BACTERIAL CELL-WALL SYNTHESIS IN MANNER SIMILAR TO THAT OF PENICILLIN. /CEPHALOTHIN & ITS CONGENERS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1160
/PENICILLINS & CEPHALOSPORINS/...HAVE SIMILAR MECHANISM OF ANTIBACTERIAL ACTION; BOTH INTERFERE WITH TERMINAL STEP IN BACTERIAL CELL WALL SYNTH BY INACTIVATING TRANSPEPTIDASE, THEREFORE PREVENTING CROSS-LINKAGE OF PEPTIDOGLYCAN CHAINS. /PENICILLINS & CEPHALOSPORINS/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 712
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
