Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
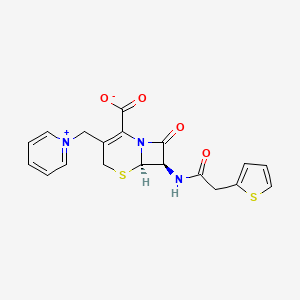

1. Cefaloridine
2. Cephalomycine
3. Cephaloridin
4. Ceporin
1. Cefaloridine
2. 50-59-9
3. Cefaloridin
4. Cephaloridin
5. Cephaloridinum
6. Cefalorizin
7. Cepaloridin
8. Cephalomycine
9. Ceflorin
10. Cepalorin
11. Loridine
12. Cefaloridina
13. Cefaloridinum
14. Kefloridin
15. Ceporin
16. Glaxoridin
17. Chebi:3537
18. N-(7-(2'-thienylacetamidoceph-3-ylmethyl))-pyridinium-2-carboxylate
19. Aliporina
20. N-(7-((2-thienyl)acetamido)ceph-3-em-3-ylmethyl)pyridinium-4-carboxylate
21. Cefaloridine [inn]
22. 7-((2-thienyl)acetamido)-3-(1-pyridylmethyl)cephalosporanic Acid
23. Intrasporin
24. Lvz1vc61hb
25. Ampligram
26. Ceporan
27. Ceporine
28. Cilifor
29. Deflorin
30. Faredina
31. Keflodin
32. Keflordin
33. Kefspor
34. Lloncefal
35. Sefacin
36. Cer
37. Betaine Cephaloridine
38. (6r,7r)-8-oxo-3-(pyridin-1-ium-1-ylmethyl)-7-[(2-thiophen-2-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
39. (6r,7r)-8-oxo-3-(pyridinium-1-ylmethyl)-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
40. Cephaloridine (usan)
41. Vioviantine
42. Sasperin
43. Verolgin
44. Sch 11527
45. Cephaloridine [usan]
46. Pyridinium, 1-((2-carboxy-8-oxo-7-((2-thienylacetyl)amino)-5-thia-1-azabicyclo(4.2.0)-oct-2-en-3-yl)methyl)-, Hydroxide, Inner Salt, (6r-trans)-
47. Lilly 40602
48. Ceph 87/4
49. Cefaloridinum [inn-latin]
50. 3-(pyridinium-1-ylmethyl)-7beta-[(2-thienylacetyl)amino]-3,4-didehydrocepham-4-carboxylate
51. Cefaloridina [inn-spanish]
52. (6r,7r)-8-oxo-3-(pyridin-1-ium-1-ylmethyl)-7-(2-(thiophen-2-yl)acetamido)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
53. Hsdb 3023
54. Einecs 200-052-6
55. Unii-lvz1vc61hb
56. Cephaloridine [usan:usp]
57. 7-(thiophene-2-acetamido)-3-(1-pyridylmethyl)-3-cephem-4-carboxylic Acid Betaine
58. (6r,7r)-1-((2-carboxy-8-oxo-7-(2-(2-thienyl)acetamido)-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)pyridinium Hydroxide, Inner Salt
59. Kefloridin (tn)
60. Ncgc00094956-01
61. Cefaloridine (jan/inn)
62. Dsstox_cid_2782
63. Cefaloridine [jan]
64. Cephaloridine [mi]
65. Pyridinium, 1-((2-carboxy-8-oxo-7-(2-(2-thienyl)acetamido)-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-, Hydroxide, Inner Salt
66. Epitope Id:120366
67. Schembl3936
68. 7-(alpha-(2-thienyl)acetamido)-3-(1-pyridylmethyl)-3-cephem-4-carboxylic Acid Betaine
69. Cephaloridine [hsdb]
70. Dsstox_rid_76727
71. Dsstox_gsid_22782
72. Cefaloridine [mart.]
73. 1-((7'-beta-(2-(2-thienyl)acetamido)-8'-oxo-1'-aza-5'-thiabicyclo(4.2.0)-oct-2'-en-3'-yl)methyl)pyridinium-2'-carboxylate
74. Pyridinium, 1-((2-carboxy-8-oxo-7-((2-thienylacetyl)amino)-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-, Inner Salt, (6r-trans)-
75. Cefaloridine [who-dd]
76. Chembl316157
77. Dtxsid9022782
78. Schembl23270209
79. Gtpl12189
80. Hy-b2072
81. Tox21_111368
82. Bdbm50103624
83. Akos016014429
84. Db09008
85. Cas-50-59-9
86. 7-(.alpha.-(2-thienyl)acetamido)-3-(1-pyridylmethyl)-3-cephem-4-carboxylic Acid Betaine
87. Cs-0017449
88. D01075
89. Q5063323
90. 7-[.alpha.(2-thienyl)-acetoamido]-3-(1-pyridylmethyl)-3-cephem-4-carboxylic Acid Betaine
91. ((6r,7r)-1-((2-carboxy-8-oxo-7-(2-(2-thienyl)acetamido)-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)pyridinium Hydroxide, Inner Salt
92. (6r,7r)-8-oxo-3-(pyridin-1-ium-1-ylmethyl)- 7-[(2-thiophen-2-ylacetyl)amino]-5-thia-1- Azabicyclo[4.2.0]oct-2-ene-2-carboxylate
93. (6r,7r)-8-oxo-3-(pyridin-1-ium-1-ylmethyl)-7-[[2-(2-thienyl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
| Molecular Weight | 415.5 g/mol |
|---|---|
| Molecular Formula | C19H17N3O4S2 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 415.06604838 g/mol |
| Monoisotopic Mass | 415.06604838 g/mol |
| Topological Polar Surface Area | 147 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 687 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cephalosporins
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
GENERAL RANGE OF ACTIVITY & ANTIBACTERIAL SPECTRUM OF CEPHALORIDINE CLOSELY APPROX THAT OF CEPHALOTHIN, ALTHOUGH SOME STRAINS OF E COLI MAY BE SOMEWHAT MORE SENSITIVE TO FORMER. IT ALSO APPEARS TO BE MORE ACTIVE THAN CEPHALOTHIN AGAINST CL PERFRINGENS (WELCHII). MYCOBACTERIUM FORTUITUM IS SENSITIVE TO CEPHALORIDINE...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1160
CEPHALORIDINE...GIVEN PARENTERALLY & SUBCONJUNCTIVALLY TO TREAT INTRAOCULAR INFECTIONS & MAY BE ADMIN TOPICALLY & SUBCONJUNCTIVALLY TO TREAT CORNEAL ULCERS.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 957
CEPHALORIDINE IS EFFECTIVE IN THERAPY OF BRONCHITIS DUE TO H INFLUENZAE, BUT OTHER AGENTS OFTEN PRODUCE BETTER RESULTS, THIS DRUG HAS ALSO BEEN FOUND USEFUL WHEN EMPLOYED AS AEROSOL IN PATIENT WITH PURULENT BRONCHITIS.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1163
For more Therapeutic Uses (Complete) data for CEPHALORIDINE (10 total), please visit the HSDB record page.
CEPHALORIDINE ACCUM IN BLOOD OF PATIENTS WITH DECR RENAL FUNCTION, & IN AZOTEMIC PATIENTS PLASMA CONCN ARE VERY HIGH; SINGLE DOSE OF 1 G IM YIELDS DETECTABLE CONCN FOR AS LONG AS 4 DAYS. ...CEPHALORIDINE SHOULD NOT BE GIVEN TO SUCH PATIENTS, SINCE IT IS NEPHROTOXIC.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
WHILE CEPHALORIDINE PRODUCES LESS IRRITATION /THAN CEPHALOTHIN/, ITS NEPHROTOXICITY OUTWEIGHS THIS ADVANTAGE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1163
IN 48-HR HUMAN INFANTS...CEPHALORIDINE...HAVE VERY LONG PLASMA T/2, & TOXIC CONCN ARE REACHED WHEN DOSES ARE LOWERED CONSIDERABLY.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 681
CEPHALORIDINE IS INJECTED EITHER IM OR IV. ... SINCE OTHER, LESS TOXIC CEPHALOSPORINS ARE AVAIL, THERE IS NO REASON TO RECOMMEND THIS PREPN.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1162
For more Drug Warnings (Complete) data for CEPHALORIDINE (7 total), please visit the HSDB record page.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DB - First-generation cephalosporins
J01DB02 - Cefaloridine
Route of Elimination
Renal.
.../CEPHALORIDINE/ POORLY ABSORBED FROM GI TRACT. PEAK PLASMA CONCN ARE REACHED ABOUT 30 MIN AFTER DRUG IS INJECTED; 10 TO 20% OF PLASMA CEPHALORIDINE IS BOUND TO PROTEIN.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
IM INJECTIONS OF 0.5 & 1 G YIELD PEAK PLASMA CONCN OF 15 & 30 UG/ML, RESPECTIVELY. APPROX 75% OF GIVEN DOSE IS EXCRETED IN URINE, MAINLY BY GLOMERULAR FILTRATION. CEPHALORIDINE ACCUM IN BLOOD OF PATIENTS WITH DECR RENAL FUNCTION, & IN AZOTEMIC PATIENTS PLASMA CONCN ARE VERY HIGH...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
PLACENTAL DRUG TRANSFER- CEPHALORIDINE: TIME TO APPEAR IN FETUS 30 MIN; TIME TO FETAL/MATERNAL CONCN EQUIL 5 HR. /FROM TABLE/
LaDu, B.N., H.G. Mandel, and E.L. Way. Fundamentals of Drug Metabolism and Disposition. Baltimore: Williams and Wilkins, 1971., p. 101
.../CEPHALORIDINE/ READILY PENETRATE NORMAL EYE FOLLOWING SYSTEMIC OR SUBCONJUNCTIVAL ADMIN...
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 957
...CEPHALORIDINE...SHOWN...TO PENETRATE INTO BONE TO VERY LIMITED EXTENT AFTER SC OR ORAL DOSES TO RATS. RATIOS OF BONE TO SERUM CONCN AVG...1:7 FOR CEPHALORIDINE...DURING 0.25-4 HR AFTER DOSING.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 452
.../CEPHALORIDINE/ PEAK PLASMA CONCN ARE REACHED ABOUT 30 MIN AFTER DRUG IS INJECTED... WHILE ITS T/2 (60 TO 90 MIN)...ONLY SMALL AMT ARE DETECTABLE AFTER 8 HR.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
/IN RATS/ RATIOS OF BONE TO SERUM CONCN AVG...1:7 FOR CEPHALORIDINE...DURING 0.25-4 HR AFTER /ORAL OR SC/ DOSING. DESPITE DIFFERENCES IN CONCN, T/2 IN BONE & SERUM WERE SIMILAR.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 452
CEPHALOTHIN & ITS CONGENERS INHIBIT BACTERIAL CELL-WALL SYNTHESIS IN MANNER SIMILAR TO THAT OF PENICILLIN. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1160
Cephaloglycin and cephaloridine are acutely toxic to the proximal renal tubule, in part because of their cellular uptake by a contraluminal anionic secretory carrier and in part through their intracellular attack on the mitochondrial transport and oxidation of tricarboxylic acid (TCA) cycle anionic substrates. Preliminary studies with cephaloglycin have provided evidence of a role of fatty acid (FA) metabolism in its nephrotoxicity, and work with cephaloridine has shown it to be a potent inhibitor of renal tubular cell and mitochondrial carnitine (Carn) transport.
PMID:7887988 Tune BM, Hsu CY; Biochem Pharmacol 49 (5): 727-34 (1995)
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?