Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
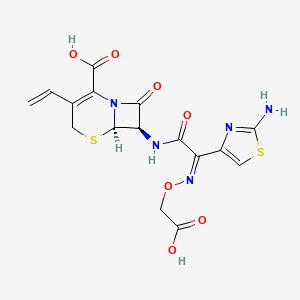

1. Cefixime Anhydrous
2. Cefixime Trihydrate
3. Fk 027
4. Fk-027
5. Fk027
6. Fr 17027
7. Fr-17027
8. Fr17027
9. Suprax
1. 79350-37-1
2. Cephoral
3. Cefixima
4. Cefiximum
5. Cefspan
6. Cefixime Anhydrous
7. Cefixim
8. Cefiximum [latin]
9. (-)-cefixim
10. Oroken
11. Cefixima [spanish]
12. Suprax
13. Fk-027
14. Cl-284635
15. Anhydrous Cefixime
16. Fk 027
17. Fr 17027
18. Fr-17027
19. Xz7bg04gjx
20. Mls002222332
21. Cefixoral
22. Chebi:472657
23. Cefixime (inn)
24. Citropen
25. Cfix
26. (6r,7r)-7-({(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(carboxymethoxy)imino]acetyl}amino)-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
27. Smr001307271
28. Unixime
29. Cefixime [inn]
30. Cl 284,635
31. Necopen
32. Denvar
33. Tricef
34. Unii-xz7bg04gjx
35. Cefixime [usan:usp:inn:ban:jan]
36. Brn 6025058
37. Cefixime [mi]
38. Cl 284635
39. (6r,7r)-7-(2-(2-amino-4-thiazolyl)glyoxylamido)-8-oxo-3-vinyl-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7(sup 2)-(z)-(o-(carboxymethyl)oxime)trihydrate
40. Prestwick3_000462
41. Cefixime [who-dd]
42. Chembl1541
43. Schembl24945
44. Bspbio_000564
45. Bpbio1_000622
46. Dtxsid7022754
47. Bdbm84007
48. Cid_5362065
49. Hms2096m06
50. Hms2234j21
51. Hms3713m06
52. (6r,7r)-7-((z)-2-(2-aminothiazol-4-yl)-2-((carboxymethoxy)imino)acetamido)-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
53. (6r,7r)-7-[[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(carboxymethoxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
54. Hy-b1381
55. Mfcd00865020
56. S4596
57. Zinc12503147
58. Akos015854940
59. Akos015961135
60. Ac-4350
61. Ccg-220462
62. Cs-4820
63. Db00671
64. Ncgc00179521-01
65. Ncgc00179521-03
66. (6r,7r)-7-(2-(2-amino-4-thiazolyl)glyoxylamido)-8-oxo-3-vinyl-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7(sup 2)-(z)-(o-(carboxymethyl)oxime) Trihydrate
67. (6r,7r)-7-[-2-(2-amino-thiazol-4-yl)-2-carboxymethoxyimino-acetylamino]-8-oxo-3-vinyl-5-thia-1-aza-b
68. (6r,7r)-7-{[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-{[(carboxymethyl)oxy]imino}acetyl]amino}-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
69. 7beta-{(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(carboxymethoxy)imino]acetamido}-3-ethenyl-3,4-didehydrocepham-4-carboxylic Acid
70. Ab00513842
71. 50c371
72. A13697
73. C-2469
74. C06881
75. D00258
76. Cefixime, Antibiotic For Culture Media Use Only
77. Sr-01000760706
78. Sr-01000760706-4
79. Brd-k71059170-001-02-5
80. Brd-k71059170-001-08-2
81. Q27290799
82. (6r,7r)-7-((2-(2-amino-1,3-thiazol-4-yl)-2-(carboxymethoxyimino)acetyl)amino)-3-ethenyl-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
83. (6r,7r)-7-[(2e)-2-(2-amino-1,3-thiazol-4-yl)-2-[(carboxymethoxy)imino]acetamido]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
84. (6r,7r)-7-[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(carboxymethoxy)imino]acetamido]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
85. (6r,7r)-7-[[(2z)-2-(2-amino-thiazol-4-yl)-2-[(carboxymethoxy)imino]acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
86. (6s,7r)-7-[[(2e)-2-(2-amino-1,3-thiazol-4-yl)-2-(carboxymethoxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
87. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2-amino-4-thiazolyl)((carboxymethoxy)imino)acetyl)amino)-3-ethenyl-8-oxo-, Trihydrate, (6r-(6alpha,7beta(z)))-
88. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2z)-(2-amino-4-thiazolyl)((carboxymethoxy)imino)acetyl)amino)-3-ethenyl-8-oxo-, (6r,7r)-
89. C04
| Molecular Weight | 453.5 g/mol |
|---|---|
| Molecular Formula | C16H15N5O7S2 |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 8 |
| Exact Mass | 453.04129018 g/mol |
| Monoisotopic Mass | 453.04129018 g/mol |
| Topological Polar Surface Area | 238 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 861 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For use in the treatment of the following infections when caused by susceptible strains of the designated microorganisms: (1) uncomplicated urinary tract infections caused by Escherichia coli and Proteus mirabilis, (2) otitis media caused by Haemophilus influenzae (beta-lactamase positive and negative strains), Moraxella catarrhalis (most of which are beta-lactamase positive), and S. pyogenes, (3) pharyngitis and tonsillitis caused by S. pyogenes, (4) acute bronchitis and acute exacerbations of chronic bronchitis caused by Streptococcus pneumoniae and Haemophilus influenzae (beta-lactamase positive and negative strains), and (5) uncomplicated gonorrhea (cervical/urethral) caused by Neisseria gonorrhoeae (penicillinase- and non-penicillinase-producing strains).
FDA Label
Cefixime, an antibiotic, is a third-generation cephalosporin like ceftriaxone and cefotaxime. Cefixime is highly stable in the presence of beta-lactamase enzymes. As a result, many organisms resistant to penicillins and some cephalosporins due to the presence of beta-lactamases, may be susceptible to cefixime. The antibacterial effect of cefixime results from inhibition of mucopeptide synthesis in the bacterial cell wall.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DD - Third-generation cephalosporins
J01DD08 - Cefixime
Absorption
About 40%-50% absorbed orally whether administered with or without food, however, time to maximal absorption is increased approximately 0.8 hours when administered with food.
Hepatic. Approximately 50% of the absorbed dose is excreted unchanged in the urine in 24 hours.
3-4 hours (may range up to 9 hours). In severe renal impairment (5 to 20 mL/min creatinine clearance), the half-life increased to an average of 11.5 hours.
Like all beta-lactam antibiotics, cefixime binds to specific penicillin-binding proteins (PBPs) located inside the bacterial cell wall, causing the inhibition of the third and last stage of bacterial cell wall synthesis. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins; it is possible that cefixime interferes with an autolysin inhibitor.
ABOUT THIS PAGE
76
PharmaCompass offers a list of CFIX API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right CFIX manufacturer or CFIX supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred CFIX manufacturer or CFIX supplier.
PharmaCompass also assists you with knowing the CFIX API Price utilized in the formulation of products. CFIX API Price is not always fixed or binding as the CFIX Price is obtained through a variety of data sources. The CFIX Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A CFIX manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of CFIX, including repackagers and relabelers. The FDA regulates CFIX manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. CFIX API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A CFIX supplier is an individual or a company that provides CFIX active pharmaceutical ingredient (API) or CFIX finished formulations upon request. The CFIX suppliers may include CFIX API manufacturers, exporters, distributors and traders.
CFIX Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of CFIX GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right CFIX GMP manufacturer or CFIX GMP API supplier for your needs.
A CFIX CoA (Certificate of Analysis) is a formal document that attests to CFIX's compliance with CFIX specifications and serves as a tool for batch-level quality control.
CFIX CoA mostly includes findings from lab analyses of a specific batch. For each CFIX CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
CFIX may be tested according to a variety of international standards, such as European Pharmacopoeia (CFIX EP), CFIX JP (Japanese Pharmacopeia) and the US Pharmacopoeia (CFIX USP).
