
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
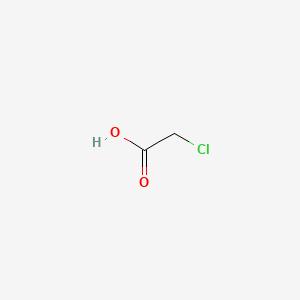

1. Acetocaustin
2. Chloroacetate
3. Chloroacetic Acid, Aluminum Salt
4. Chloroacetic Acid, Ammonium (2:1) Salt
5. Chloroacetic Acid, Ammonium Salt
6. Chloroacetic Acid, Calcium (3:1) Salt
7. Chloroacetic Acid, Calcium Salt
8. Chloroacetic Acid, Potassium (2:1) Salt
9. Chloroacetic Acid, Potassium Salt
10. Chloroacetic Acid, Silver Salt
11. Chloroacetic Acid, Sodium (2:1) Salt
12. Chloroacetic Acid, Sodium (5:2) Salt
13. Chloroacetic Acid, Sodium Salt
14. Monochloroacetic Acid
15. Sodium Chloroacetate
1. Monochloroacetic Acid
2. 79-11-8
3. 2-chloroacetic Acid
4. Chloracetic Acid
5. Chloroethanoic Acid
6. Acetic Acid, Chloro-
7. Acide Chloracetique
8. Monochloroethanoic Acid
9. Monochloracetic Acid
10. Monochloorazijnzuur
11. Monochloressigsaeure
12. Acidomonocloroacetico
13. Chloroacetic Acid, Solid
14. Chloroacetic
15. 2-chloro-acetic Acid
16. Acide Monochloracetique
17. Chloroacetic Acid [bsi:iso]
18. Acetic Acid, 2-chloro-
19. .alpha.-chloroacetic Acid
20. Acide Chloroacetique
21. Kyselina Chloroctova
22. Monochloorazijnzuur [dutch]
23. Acide Chloracetique [french]
24. Kyselina Chloroctova [czech]
25. Monochloressigsaeure [german]
26. Acide Chloroacetique [french]
27. Alpha-chloroacetic Acid
28. 2-chloro-ethanoic Acid
29. Acidomonocloroacetico [italian]
30. Acide Monochloracetique [french]
31. Chloroacetic Acid, Molten
32. Monochloroacetic Acid [bsi:iso]
33. Nsc 142
34. Acide Chloracetique [iso-french]
35. Chloroacetic Acid Solution
36. Chloroacetic Acid (80% Or Less)
37. Nci-c60231
38. Chloro-acetic Acid
39. Acetocaustin (tn)
40. Ch2clcooh
41. Monochloro Acetic Acid
42. Chloroacetic-acid
43. Monochloracetic Acidacide Monochloracetique
44. Monochloroacetic Acid, Solution
45. Chloroacetic Acid, Liquid
46. Chebi:27869
47. Nsc-142
48. 5gd84y125g
49. Mfcd00002683
50. Chloroacetic Acid Crystalline
51. Chloroacetic Acid, Molten [un3250] [poison]
52. Chloroacetic Acid, Solid [un1751] [poison]
53. Chloroacetic Acid, Solution [un1750] [poison]
54. Acetocaustin
55. Caswell No. 179b
56. Sjphlqdiktp@
57. Ccris 2117
58. Hsdb 939
59. Einecs 201-178-4
60. Un1750
61. Un1751
62. Un3250
63. Epa Pesticide Chemical Code 279400
64. Brn 0605438
65. Chloroacetic Acid, Acs
66. Unii-5gd84y125g
67. Cloroacetic Acid
68. Ai3-25035
69. Chloro Acetic Acid
70. A-chloroacetic Acid
71. R3w
72. Clch2cooh
73. Monochloro-acetic Acid
74. Iso-alkanesc13-c16
75. Alpha-chloro-acetic Acid
76. Chloroacetic Acid, 99%
77. Dsstox_cid_901
78. Chloroacetic Acid, Solid [un1751] [poison]
79. Bmse000367
80. Wln: Qv1g
81. Chloroacetic Acid, Molten [un3250] [poison]
82. Chloroacetic Acid, Solution
83. Ec 201-178-4
84. Chloroacetic Acid, >=99%
85. Dsstox_rid_75855
86. Nciopen2_002217
87. Dsstox_gsid_20901
88. 4-02-00-00474 (beilstein Handbook Reference)
89. Mls001065621
90. Bidd:er0630
91. Chembl14090
92. Chloroacetic Acid [mi]
93. Nsc142
94. Chloroacetic Acid [iso]
95. Chloroacetic Acid, Solid (dot)
96. Chloroacetic Acid [hsdb]
97. Chloroacetic Acid [inci]
98. Dtxsid4020901
99. Focautsvdikzop-uhfffaoysa-
100. Chloroacetic Acid [vandf]
101. Bcp20585
102. Nsc42970
103. Str00326
104. Zinc3860254
105. Tox21_201114
106. Bbl037260
107. Lmfa01090068
108. Monochloroacetic Acid [vandf]
109. Nsc-42970
110. Stl197882
111. Chloroacetic Acid, Analytical Standard
112. Monochloroacetic Acid [mart.]
113. Akos000118920
114. Monochloroacetic Acid [who-dd]
115. Un 1751
116. Cas-79-11-8
117. Ncgc00091473-01
118. Ncgc00091473-02
119. Ncgc00091473-03
120. Ncgc00258666-01
121. Smr000568484
122. Chloroacetic Acid, For Synthesis, 99.0%
123. Chloroacetic Acid, Acs Reagent, >=99.0%
124. Chloroacetic Acid, Purum, >=97.0% (t)
125. Chloroacetic Acid, Puriss., >=99.0% (t)
126. C06755
127. D07677
128. Chloroacetic Acid, Jis Special Grade, >=99.0%
129. Q409013
130. J-520023
131. Chloroacetic Acid, Pestanal(r), Analytical Standard
132. F2190-0289
133. Chloroacetic Acid 1000 Microg/ml In Methyl-tert-butyl Ether
| Molecular Weight | 94.50 g/mol |
|---|---|
| Molecular Formula | C2H3ClO2 |
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 93.9821570 g/mol |
| Monoisotopic Mass | 93.9821570 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 42.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Cryotherapy and salicylic acid (SA) often fail as treatments for skin warts. We examined the effectiveness of monochloroacetic acid (MCA) for patients with common or plantar warts. Consecutive patients aged 4 years and older with one or more newly diagnosed common or plantar warts were recruited in 53 Dutch general practices. We randomly allocated eligible patients to 13-week treatment protocols of office-applied MCA versus liquid nitrogen cryotherapy every 2 weeks for patients with common warts (n=188), and MCA versus cryotherapy combined with daily SA self-application for patients with plantar warts (n=227). The primary outcome was the proportion of patients whose warts were all cured at 13 weeks. In the common wart group, cure rates were 40/92 (43%, 95% confidence interval 34-54) for MCA and 50/93 (54%, 44-64) for cryotherapy (risk difference (RD) -10%, -25-4.0, P=0.16). In the plantar wart group, cure rates were 49/106 (46%, 37-56) for MCA and 45/115 (39%, 31-48) for cryotherapy combined with SA (RD 7.1, 5.9-20, P=0.29). For common warts, MCA is an effective alternative to cryotherapy to avoid pain during the treatment, although pain after the treatment is similar. For plantar warts, office-applied MCA may be preferred over cryotherapy combined with SA, on the basis of comparable effectiveness, less treatment pain, and less treatment burden.
PMID:25584800 Bruggink SC et al; J Invest Dermatol 135 (5): 1261-1267 (2015)
Probable Oral Lethal Dose (human): 50-500 mg/kg, between 1 teaspoon and 1 o z for 70 kg person (150 lb).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-198
Distribution, metabolism, and excretion of monochloroacetic acid (MCA) were examined in adult male rats at a subtoxic (10 mg/kg) and a toxic (75 mg/kg) dose. Rats were injected i.v. with 14(C)MCA and housed individually. Urine and feces were collected. ... Radioactivity in aliquots showed very rapid distribution of MCA to tissues. Concentrations of MCA in plasma, liver, heart, lungs, and brown fat paralleled each other, whereas those in brain and thymus did not. There was no dose proportionality in tissue concentrations. Elimination of MCA from plasma required modeling by two compartments. Most of the radioactivity found in plasma was parent MCA. Elimination rate constant (K(10)) and distribution rate constant (K(12)) were greatly reduced at the toxic dose. Elimination of the toxic dose was further retarded due to increased retention of MCA in the peripheral compartment as indicated by increased mean residence times in most tissues. A very large fraction of dose was found in the gastrointestinal tract, almost all of which was reabsorbed. Attempts to reduce toxicity by blocking the enterohepatic circulation with activated charcoal or cholestyramine failed. Radioactivity found in bile was associated with one metabolite more polar than the parent compound. A very large fraction of dose (73 and 59%) was found in urine, 55 to 68% of which was parent MCA. The rate-determining step in the toxicity of MCA was identified as its detoxification by the liver...
PMID:11160650 Saghir SA et al; J Pharmacol Exp Ther 296 (2): 612-22 (2001)
Rats were administered a single oral (10 (subtoxic) or 225 (toxic, LD20) mg/kg) or dermal (125 mg/kg, LD20) dose of (14)C-monochloroacetic acid (MCA) and the time-course (0.25, 0.75, 2, 4, 8, 16, and 32 hr postadministration) of radioactivity was determined in plasma, tissues, and excreta. At the subtoxic oral dose, concentration of (14)C-MCA peaked at 0.1% of dose by 2 hr. Most tissue profiles of MCA paralleled that of plasma with few exceptions. At the toxic oral dose, tissue concentrations remained initially below those seen after the subtoxic dose, because stomach retained most of the toxic dose for up to 8 hr. Peak plasma concentration was reached within 0.25 hr without an apparent subsequent uptake phase. Most of the dermal dose rapidly penetrated into the skin (>95% within 0.25 hr) and remained sequestered there and released slowly. Concentration in plasma peaked at 0.36% of dose by 0.75 hr and remained constant for up to 4 hr. Peak tissue concentrations were reached between 2 and 4 hr. Within 0.75 hr, 9% of the dermally absorbed dose was metabolized by liver and eliminated through bile, all of which was subsequently reabsorbed. Two percent of MCA appeared in colon by 0.75 hr, apparently as a result of direct transport through GI-wall in retrograde movement. About 70-80% of radioactivity recovered from the small intestine of orally dosed rats was parent compound. Fecal elimination was negligible ( 400 and < 450) and 175 (LD50 145) mg/kg after oral and dermal exposure, respectively.
PMID:12915718 Saghir SA, Rozman KK; Toxicol Sci 76 (1): 51-64 (2003)
Distribution of monochloroacetic acid (CA) was studied in rats given a single oral dose of 0.1 mmole/kg body weight [1-(14)C]CA, by gavage. The animals were sacrificed at 4, 8, 12, 24 and 48 hr following the treatment. The distribution of (14)C-label, determined in different tissues, suggests that CA is rapidly absorbed and eliminated from the body. The elimination phase appears to be fast for intestine and kidney as compared to other tissues. Maximum radioactivity was detected in intestine and kidney at 4 and 8 hr following the treatment which was followed by liver, spleen, testes, lung, brain and heart in a decreasing order. A group of rats treated with a single oral dose of 1 mmole/kg (1-(14)C)CA, by gavage, was also sacrificed at 24 hr following the exposure to study the effect of a higher dose on distribution of (1-(14)C)CA. The distribution of (14)C-label at both dose levels indicates that toxicokinetic properties of CA are dose-dependent. Another group of rats administered 1 mmole/kg (1-(14)C) CA daily for three days was also sacrificed at 24 hr following the last dose to evaluate the bioaccumulating properties of CA and/or its metabolites in the tissues. As compared to the number of doses given, the accumulation of (14)C-label was not as large as expected. (14)C-Label determined in the dialyzed plasma, suggests an in vivo binding of (14)C-label to plasma proteins where albumin accounted for about 65% as determined by affinity chromatography...
PMID:1542885 Kaphalia BS et al; Toxicol Ind Health 8 (1-2): 53-61 (1992)
... To understand the mechanism of monochloroacetic acid (MCA) toxicity, ... the tissue distribution of (1-(14)C)MCA in rats /was studied/ by a whole-body autoradiographic technique. Male Sprague-Dawley rats were given a tracer dose of (1-14C)MCA (6.8 ug/100 g (40 uCi) body weight) by tail vein and euthanized at different time intervals (5 min, 1, 4, 12, 24 and 48 hr). ... At 5 min, there was a rapid accumulation of (14)C-activity in the kidney cortex and stomach walls. The radioactivity was rapidly removed from the circulation. There was high accumulation of (14)C-activity in the myocardial tissues. The liver was also loaded with MCA and/or its metabolites. After 1 hr following administration of (14)MCA, radioactivity was extensively excreted into the small intestinal lumen. The accumulation of (14)C-activity in the brain, thymus, salivary glands and tongue was prominent at 1 hr. After 4 hr the liver and other tissues started to eliminate most of the radioactivity. Contrary to other tissues, however, the central nervous system, thymus and pancreas started to accumulate the radioactivity at later time periods. These observations suggest the accumulation of MCA and/or its metabolites into hydrophilic tissues at earlier time periods and into lipophilic tissues at later times.
PMID:2116687 Bhat HK et al; Toxicology 63 (1): 35-43 (1990)
For more Absorption, Distribution and Excretion (Complete) data for Chloroacetic acid (13 total), please visit the HSDB record page.
The metabolism of 14(C)-dichloroethyne was studied in rats by inhalation in a dynamic nose-only exposure system. ... Metabolites of dichloroethyne identified are: N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine, dichloroethanol, dichloroacetic acid, oxalic acid and chloroacetic acid in urine; N-acetyl-S-(1,2-dichlorovinyl-L-cysteine in feces...
PMID:1776266 Kanhai W et al; Xenobiotica 21 (7): 905-16 (1991)
The metabolism of MCA has been characterised in the mouse following MCA administration by intraperitoneal injection. Metabolites of MCA identified in the urine included S-carboxymethylcysteine (33%-43% free and 1%-6% conjugated), thiodiacetic acid (thiodiglycolic acid was found to be the major urinary metabolite of S-carboxymethylcysteine, and most of the glycolate was oxidized to carbon dioxide. The metabolism proceeds probably by enzymatic hydrolysis of the carbon-chlorine bond with the formation of glycolic acid . MCA also conjugates with GSH to form the S-carboxymethyl derivative of GSH, which is then converted to S-carboxymethyl cysteine. In Wistar rats given 50 mg/kg MCA by gavage, thiodiglycolic acid was identified as the major urinary metabolite, accounting for 60% of the administered dose. A greater percentage of administered MCA was excreted as thiodiglycolic acid in rats than in mice; in both species most of the remainder of the dose was excreted as S-carboxymethylcysteine. ... Preliminary pharmacokinetic studies with MCA in rats /showed/ that MCA was rapidly metabolized, and detoxication by conjugation with glutathione appeared to be a major metabolic pathway.
OECD; IRPTC Data Profile/SIDS Dataset for Chloroacetic Acid (CAS No: 79-11-8) p.60 (December 13, 2005) Available from, as of November 28, 2018: https://www.inchem.org/documents/sids/sids/79118.pdf
... A 17-year-old male ... ingested approximately 70 mL trichloroethene (TRI) in a suicide attempt. ... During /5 days/ ... blood and urine were collected and TRI and its metabolites were quantified. ... Trichloroethanol and trichloroacetic acid, metabolites of the cytochrome P450-mediated pathway, and N-acetyl-S-(1, 2-dichlorovinyl)-l-cysteine and N-acetyl-S-(2, 2-dichlorovinyl)-l-cysteine from the glutathione-dependent pathway of TRI were quantified in urine samples. Besides these known metabolites in humans, chloroacetic acid and dichloroacetic acid were identified ... in urine of a human exposed to TRI ...
PMID:9520351 Bruning T et al; Toxicol Sci 41 (2): 157-65 (1998)
In one human case the majority of MCA was excreted as nonmetabolized MCA. A minor part reacted with glutathione and was excreted in urine as the conjugate. A small amount was metabolized and excreted as carbon dioxide in exhaled air ... .
IPCS; Poisons Information Monograph 352: Monochloroacetic acid (2000). Available from, as of November 28, 2018: https://www.inchem.org/pages/pims.html
For more Metabolism/Metabolites (Complete) data for Chloroacetic acid (6 total), please visit the HSDB record page.
... A case of human skin contamination with hot chloroacetic acid labelled with carbon-14 /was reported/. ... A half-life of about 15 hours was found for the excretion of MCAA in urine. ...
ECB/ESIS; European Union Risk Assessment Report: MONOCHLOROACETIC ACID (MCAA) (CAS-No 79-11-8) p.64 (2005) 3rd Priority List Vol 52. Available from, as of November 27, 2018: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/european-union-risk-assessment-report-monochloroacetic-acid-mcaa-cas-no-79-11-8-einecs-no
... Plasma disappearance of radioactivity, given at ... 53 mg/kg was biphasic (approximately rapid phase half-life: 90 minutes; slow phase: 500 minutes). ...
ECB/ESIS; European Union Risk Assessment Report: MONOCHLOROACETIC ACID (MCAA) (CAS-No 79-11-8) p.60 (2005) 3rd Priority List Vol 52. Available from, as of November 27, 2018: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/european-union-risk-assessment-report-monochloroacetic-acid-mcaa-cas-no-79-11-8-einecs-no
... Male Swiss-Webster mice (n = 6/dose group) were treated orally with a single dose of 0.6, 150, or 250 mg/kg of (1-(14)C)-MCAA (1.0 uCi). It was found that MCAA is rapidly absorbed by the oral route, rapidly eliminated from the body with a half-life not exceeding 12 hours in non-nervous tissues and 26 hours in the CNS. ...
ECB/ESIS; European Union Risk Assessment Report: MONOCHLOROACETIC ACID (MCAA) (CAS-No 79-11-8) p.59 (2005) 3rd Priority List Vol 52. Available from, as of November 27, 2018: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/european-union-risk-assessment-report-monochloroacetic-acid-mcaa-cas-no-79-11-8-einecs-no
Rats were administered a single oral (10 (subtoxic) or 225 (toxic, LD20) mg/kg) or dermal (125 mg/kg, LD20) dose of (14)C-monochloroacetic acid (MCA) and the time-course (0.25, 0.75, 2, 4, 8, 16, and 32 hr postadministration) of radioactivity was determined in plasma, tissues, and excreta. ... . The plasma half-life was 2 hr for oral and 4 hr for dermal administration. ...
PMID:12915718 Saghir SA, Rozman KK; Toxicol Sci 76 (1): 51-64 (2003)
In vitro, MCA blocks the cell energy supply in an as yet incompletely resolved manner, leading to a gradual decrease in ATP generation and in protein synthesis. Supplementation of intermediates of the Krebs-cycle or of acetyl-donors does not reduce this effect whereas incubation with the sodium salt of MCA causes a slow but marked decrease in the activity of pyruvate dehydrogenase and to a lesser degree of keto-glutarate dehydrogenase ... .
IPCS; Poisons Information Monograph 352: Monochloroacetic acid (2000). Available from, as of November 28, 2018: https://www.inchem.org/pages/pims.html
ANALYTICAL

ABOUT THIS PAGE
92
PharmaCompass offers a list of Chloroacetic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Chloroacetic Acid manufacturer or Chloroacetic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Chloroacetic Acid manufacturer or Chloroacetic Acid supplier.
PharmaCompass also assists you with knowing the Chloroacetic Acid API Price utilized in the formulation of products. Chloroacetic Acid API Price is not always fixed or binding as the Chloroacetic Acid Price is obtained through a variety of data sources. The Chloroacetic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Chloroacetic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Chloroacetic Acid, including repackagers and relabelers. The FDA regulates Chloroacetic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Chloroacetic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Chloroacetic Acid supplier is an individual or a company that provides Chloroacetic Acid active pharmaceutical ingredient (API) or Chloroacetic Acid finished formulations upon request. The Chloroacetic Acid suppliers may include Chloroacetic Acid API manufacturers, exporters, distributors and traders.
Chloroacetic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Chloroacetic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Chloroacetic Acid GMP manufacturer or Chloroacetic Acid GMP API supplier for your needs.
A Chloroacetic Acid CoA (Certificate of Analysis) is a formal document that attests to Chloroacetic Acid's compliance with Chloroacetic Acid specifications and serves as a tool for batch-level quality control.
Chloroacetic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Chloroacetic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Chloroacetic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Chloroacetic Acid EP), Chloroacetic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Chloroacetic Acid USP).
