



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
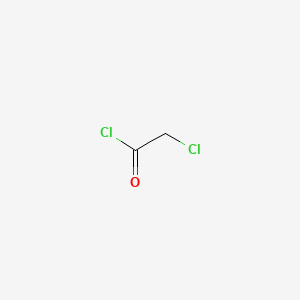

1. Monochloroacetyl Chloride
1. 79-04-9
2. 2-chloroacetyl Chloride
3. Acetyl Chloride, Chloro-
4. Chloracetyl Chloride
5. Chloroacetic Chloride
6. Monochloroacetyl Chloride
7. Chloroacetylchloride
8. Chloroacetic Acid Chloride
9. Chlorure De Chloracetyle
10. Chloracetylchloride
11. Chlorid Kyseliny Chloroctove
12. Chloro Acetyl Chloride
13. Chloro-acetyl Chloride
14. Chloroethanoyl Chloride
15. K5uml06yuo
16. Acetyl Chloride, 2-chloro-
17. Monochloroacetic Acid Chloride
18. .alpha.-chloroacetyl Chloride
19. Chebi:34624
20. Mfcd00000725
21. Ccris 9145
22. Hsdb 973
23. Chlorure De Chloracetyle [french]
24. Chlorid Kyseliny Chloroctove [czech]
25. Einecs 201-171-6
26. Unii-k5uml06yuo
27. Un1752
28. Brn 0605439
29. Chloroacetylchlonde
30. Chloroacetylchoride
31. Chloroactylchloride
32. Chloroacetylchlorid
33. Chloro Acetylchlorid
34. Chloroacetyl Choride
35. Chloroactyl Chloride
36. Choroacetyl Chloride
37. Chioroacetyl Chloride
38. Chloro-acetylchloride
39. Chloroacetyl-chloride
40. Chloro Acetylchloride
41. Ch2clcocl
42. Clch2cocl
43. Chloro-actyl Chloride
44. Chloroacethyl Chloride
45. 2-chloroacetylchloride
46. Chloroacetyl Chloride-
47. A-chloroacetyl Chloride
48. 2-chloro Acetylchloride
49. 2-chloro-acetylchloride
50. 2-chloroacetic Chloride
51. Alpha-chloroacetylchloride
52. 2-chloro-acetyl Chloride
53. Alpha-chloro-acetylchloride
54. Dsstox_cid_6472
55. Alpha-chloroacetyl Chloride
56. Ec 201-171-6
57. 2-chloranylethanoyl Chloride
58. Schembl9584
59. 2-chloroacetic Acid Chloride
60. Chloroacetyl Chloride, 98%
61. Dsstox_rid_78120
62. Dsstox_gsid_26472
63. Chembl3187685
64. Dtxsid4026472
65. Chloracetyl Chloride [mi]
66. Amy40193
67. Bcp25293
68. Zinc3860850
69. Chloroacetyl Chloride [hsdb]
70. Tox21_200644
71. Bbl013127
72. Stk399762
73. Akos000121312
74. Un 1752
75. Cas-79-04-9
76. Ncgc00248777-01
77. Ncgc00258198-01
78. Vs-03683
79. Chloroacetyl Chloride [un1752] [poison]
80. Chloroacetyl Chloride, Purum, >=99.0% (gc)
81. A839561
82. Q411258
83. J-520024
84. F2190-0033
| Molecular Weight | 112.94 g/mol |
|---|---|
| Molecular Formula | C2H2Cl2O |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 111.9482701 g/mol |
| Monoisotopic Mass | 111.9482701 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 42.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Oxidation of the vinyl halide carcinogen and hepatotoxin vinylidene chloride (VDC) by microsomal cytochrome p450 yields 2,2-dichloroacetaldehyde, 2-chloroacetyl chloride, 2-chloroacetic acid, and l,l-dichloroethylene oxide. The roles of these metabolites in covalent modification of proteins and reduced glutathione (GSH) were examined. 2-Chloroacetyl chloride reacted with model thiols at least 10(3)-fold faster than did l,l-dichloroethylene oxide and at least 10(5)-fold faster than did 2,2-dichloroacetaldehyde or 2-chloroacetic acid.
PMID:3965130 Liebler DC et al; Cancer Res 45 (1): 186-93 (1985)


