Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
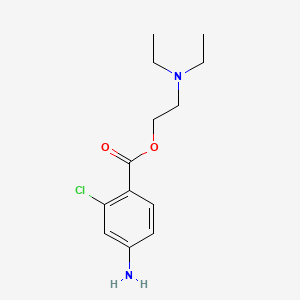

1. 2-chloroprocaine
2. Chlor-procaine
3. Chloroprocaine Hydrochloride
4. Nesacaine
5. Nesacaine Mpf
1. Chloroprocain
2. 2-chloroprocaine
3. 133-16-4
4. Chlorprocaine
5. Halestyn
6. 2-(diethylamino)ethyl 4-amino-2-chlorobenzoate
7. Piocaine
8. Chloroprocainum
9. Cloroprocaina
10. Chloroprocaine [inn]
11. Nesacaine
12. Benzoic Acid, 4-amino-2-chloro-, 2-(diethylamino)ethyl Ester
13. 2-diethylaminoethyl 4-amino-2-chloro-benzoate
14. Chloroprocaine (inn)
15. 5yvb0pot2h
16. Chlor-procaine
17. 4-amino-2-chlorobenzoic Acid 2-(diethylamino)ethyl Ester
18. Chebi:3636
19. Chlorprocainum
20. Chloroprocainum [inn-latin]
21. Cloroprocaina [inn-spanish]
22. Hsdb 3301
23. Unii-5yvb0pot2h
24. 2-(diethylaminoethyl)-4-amino-2-chlorobenzoate
25. Brn 2808071
26. Chloroprocaine [mi]
27. Schembl6676
28. Chloroprocaine [hsdb]
29. 4-14-00-01273 (beilstein Handbook Reference)
30. Chloroprocaine [vandf]
31. Gtpl7145
32. Chloroprocaine [who-dd]
33. Chembl1179047
34. Dtxsid8022799
35. Hy-b1604a
36. Zinc1530938
37. Akos010575135
38. Db01161
39. Ncgc00183273-04
40. Cs-0013537
41. Ft-0713386
42. C07877
43. D07678
44. Sr-01000944416
45. Q2964133
46. Sr-01000944416-1
| Molecular Weight | 270.75 g/mol |
|---|---|
| Molecular Formula | C13H19ClN2O2 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 270.1135055 g/mol |
| Monoisotopic Mass | 270.1135055 g/mol |
| Topological Polar Surface Area | 55.6 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 259 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Chloroprocaine hydrochloride |
| PubMed Health | Chloroprocaine (Injection) |
| Drug Classes | Anesthetic, Local |
| Drug Label | Nesacaine and Nesacaine-MPF Injections are sterile non pyrogenic local anesthetics. The active ingredient in Nesacaine and Nesacaine-MPF Injections is chloroprocaine HCL (benzoic acid, 4-amino-2-chloro-2-(diethylamino) ethyl ester, monohydrochloride)... |
| Active Ingredient | Chloroprocaine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2%; 3% |
| Market Status | Prescription |
| Company | Hospira; Eurohlth Intl |
| 2 of 4 | |
|---|---|
| Drug Name | Nesacaine |
| Drug Label | Nesacaine and Nesacaine-MPF Injections are sterile non pyrogenic local anesthetics. The active ingredient in Nesacaine and Nesacaine-MPF Injections is chloroprocaine HCL (benzoic acid, 4-amino-2-chloro-2-(diethylamino) ethyl ester, monohydrochloride)... |
| Active Ingredient | Chloroprocaine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1%; 2% |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 3 of 4 | |
|---|---|
| Drug Name | Chloroprocaine hydrochloride |
| PubMed Health | Chloroprocaine (Injection) |
| Drug Classes | Anesthetic, Local |
| Drug Label | Nesacaine and Nesacaine-MPF Injections are sterile non pyrogenic local anesthetics. The active ingredient in Nesacaine and Nesacaine-MPF Injections is chloroprocaine HCL (benzoic acid, 4-amino-2-chloro-2-(diethylamino) ethyl ester, monohydrochloride)... |
| Active Ingredient | Chloroprocaine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2%; 3% |
| Market Status | Prescription |
| Company | Hospira; Eurohlth Intl |
| 4 of 4 | |
|---|---|
| Drug Name | Nesacaine |
| Drug Label | Nesacaine and Nesacaine-MPF Injections are sterile non pyrogenic local anesthetics. The active ingredient in Nesacaine and Nesacaine-MPF Injections is chloroprocaine HCL (benzoic acid, 4-amino-2-chloro-2-(diethylamino) ethyl ester, monohydrochloride)... |
| Active Ingredient | Chloroprocaine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1%; 2% |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
Anesthetics, Local
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
A LOCAL ANESTHETIC AGENT SIMILAR IN CHEM STRUCTURE & PHARMACOLOGICAL ACTION TO PARENT COMPD, PROCAINE HCL. IT IS MORE RAPID IN ONSET OF ACTION & HAS ANESTHETIC POTENCY AT LEAST TWICE THAT OF PROCAINE. TOXICITY IS LOW & QUALITATIVELY SIMILAR TO PROCAINE. /CHLOROPROCAINE HCL/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 988
...USED TO PRODUCE ANESTHESIA BY TECHNIQUES OF INFILTRATION, FIELD BLOCK, & REGIONAL NERVE BLOCK, INCL CAUDAL & EPIDURAL BLOCK. /CHLOROPROCAINE HCL/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 988
... ACT ON ANY PART OF THE NERVOUS SYSTEM & ON EVERY TYPE OF NERVE FIBER. /LOCAL ANESTHETICS/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 331
For more Therapeutic Uses (Complete) data for CHLOROPROCAINE (13 total), please visit the HSDB record page.
A FEW CASES OF THROMBOPHLEBITIS HAVE BEEN REPORTED FOLLOWING IV REGIONAL ANESTHESIA. THIS REACTION MAY HAVE BEEN DUE TO ACIDITY OF SOLN. /CHLOROPROCAINE HCL/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 272
... NOT EFFECTIVE TOPICALLY & HAS NOT BEEN STUDIED SUFFICIENTLY TO BE USED FOR SPINAL ANESTHETIC. /CHLOROPROCAINE HCL/
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 165
IN PRESENCE OF HEMORRHAGE, SYMPATHETIC BLOCK PRODUCED BY EPIDURAL ANESTHESIA BECOMES EXTREMELY SIGNIFICANT & MAY RESULT IN RAPID & DELETERIOUS CIRCULATORY CHANGES. /LOCAL ANESTHETICS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 399
ANIMALS WITH EXPTL PRODUCED HEPATIC DAMAGE ARE MUCH MORE SUSCEPTIBLE TO TOXIC ACTIONS OF LOCAL ANESTHETICS, SO THAT EXTENSIVE USE OF LOCAL ANESTHETIC IN PT WITH SEVERE HEPATIC DAMAGE SHOULD PERHAPS BE AVOIDED. /LOCAL ANESTHETICS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 386
For more Drug Warnings (Complete) data for CHLOROPROCAINE (19 total), please visit the HSDB record page.
For the production of local anesthesia by infiltration and peripheral nerve block. They are not to be used for lumbar or caudal epidural anesthesia.
FDA Label
Chloroprocaine is an ester type anesthetic agent indicated for production of local or regional anesthesia, particularly for oral surgery. Chloroprocaine (like cocaine) has the advantage of constricting blood vessels which reduces bleeding, unlike other local anesthetics like lidocaine.
Anesthetics, Local
Drugs that block nerve conduction when applied locally to nerve tissue in appropriate concentrations. They act on any part of the nervous system and on every type of nerve fiber. In contact with a nerve trunk, these anesthetics can cause both sensory and motor paralysis in the innervated area. Their action is completely reversible. (From Gilman AG, et. al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed) Nearly all local anesthetics act by reducing the tendency of voltage-dependent sodium channels to activate. (See all compounds classified as Anesthetics, Local.)
N - Nervous system
N01 - Anesthetics
N01B - Anesthetics, local
N01BA - Esters of aminobenzoic acid
N01BA04 - Chloroprocaine
Absorption
The rate of systemic absorption of local anesthetic drugs is dependent upon the total dose and concentration of drug administered, the route of administration, the vascularity of the administration site, and the presence or absence of epinephrine in the anesthetic injection.
Route of Elimination
Chloroprocaine is rapidly metabolized in plasma by hydrolysis of the ester linkage by pseudocholinesterase. Urinary excretion is affected by urinary perfusion and factors affecting urinary pH.
PROCAINE IS READILY ABSORBED FOLLOWING PARENTERAL ADMIN ... DOES NOT LONG REMAIN @ SITE OF INJECTION. ... FOLLOWING ABSORPTION, PROCAINE IS RAPIDLY HYDROLYZED ... /PROCAINE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 388
... Binding of the anesthetic to proteins in the serum and to tissues reduces the concentration of free drug in the systemic circulation and, consequently, reduces toxicity. ... /Ester local anesthetics/ are hydrolyzed and inactivated primarily by a plasma esterase, probably plasma cholinesterase. The liver also participates in hydrolysis of local anesthetics. /Local anesthetics/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 338
THE SYSTEMIC TOXICITY OF CHLOROPROCAINE IS LESS THAN THAT OF ALL OTHER LOCAL ANESTHETICS BECAUSE OF ITS RAPID HYDROLYSIS BY PLASMA CHOLINESTERASE ... WHICH SHORTENS THE PLASMA HALF-LIFE.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 165
... ENZYMATIC HYDROLYSIS OF PROCAINE /GIVES/ ... PARA-AMINOBENZOIC ACID & DIETHYLAMINOETHANOL. FORMER IS EXCRETED IN URINE TO EXTENT OF ABOUT 80%, EITHER UNCHANGED OR IN CONJUGATED FORM. ONLY 30% OF DIETHYLAMINOETHANOL CAN BE RECOVERED IN URINE; REMAINDER UNDERGOES METABOLIC DEGRADATION ... /PROCAINE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 388
For more Absorption, Distribution and Excretion (Complete) data for CHLOROPROCAINE (9 total), please visit the HSDB record page.
Chloroprocaine is rapidly metabolized in plasma by hydrolysis of the ester linkage by pseudocholinesterase.
2-DIETHYLAMINOETHYL 4-AMINO-2-CHLOROBENZOATE YIELDS 4-AMINO-2-CHLOROBENZOIC ACID IN GUINEA PIGS. LIVETT, BH & RM LEE, BIOCHEM PHARMAC 17, 385 (1968). /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. D-27
HYDROLYZED /CHIEFLY/ BY PLASMA PSEUDOCHOLINESTERASES /& ALSO BY ESTERASES IN LIVER/ AS DIETHYLAMINOETHANOL & 2-CHLORO-4-AMINOBENZOIC ACID /HUMAN, PARENTERAL. ANIMAL STUDIES SUGGEST THAT SOME LOCAL ANESTHETICS MAY UNDERGO BILIARY RECYCLING/ /CHLOROPROCAINE HCL/
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1974
21 +/- 2 seconds
... Plasma half-life /is/ approximately 25 seconds.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 339
Chloroprocaine acts mainly by inhibiting sodium influx through voltage gated sodium channels in the neuronal cell membrane of peripheral nerves. When the influx of sodium is interrupted, an action potential cannot arise and signal conduction is thus inhibited. The receptor site is thought to be located at the cytoplasmic (inner) portion of the sodium channel. It is hypothesized that Chloroprocaine binds or antagonizes the function of N-methyl-D-aspartate (NMDA) receptors as well as nicotinic acetylcholine receptors and the serotonin receptor-ion channel complex.
Local anesthetics prevent the generation and the conduction of the nerve impulse. Their primary site of action is the cell membrane. ... Local anesthetics block conduction by decreasing or preventing the large transient increase in the permeability of excitable membranes to Na+ that normally is produced by a slight depolarization of the membrane. ... As the anesthetic action progressively develops in a nerve, the threshold for electrical excitability gradually increases, the rate of rise of the action potential declines, impulse conduction slows, and the safety factor for conduction decreases; these factors decrease the probability of propagation of the action potential, and nerve conduction fails. ... /Local anesthetics/ can block K+ channels. ... blockade of conduction is not accompanied by any large or consistent change in resting membrane potential due to block of K+ channels. /Local anesthetics/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 332
... SITE AT WHICH LOCAL ANESTHETICS ACT, AT LEAST IN ... CHARGED FORM, IS ACCESSIBLE ONLY FROM THE INNER SURFACE OF THE MEMBRANE. ... LOCAL ANESTHETICS APPLIED EXTERNALLY FIRST MUST CROSS THE MEMBRANE BEFORE THEY CAN EXERT A BLOCKING ACTION. /LOCAL ANESTHETICS/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 332
... /TWO POSSIBILITIES:/ ACHIEVE BLOCK BY INCR SURFACE PRESSURE OF LIPID LAYER THAT CONSTITUTES NERVE MEMBRANE ... CLOSING PORES THROUGH WHICH IONS MOVE. ... /OR:/ AFFECT PERMEABILITY BY INCR DEGREE OF DISORDER OF MEMBRANE. /LOCAL ANESTHETICS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 382
... ACID SALT MUST BE NEUTRALIZED IN TISSUE & FREE AMINE LIBERATED BEFORE DRUG CAN PENETRATE TISSUES & PRODUCE ANESTHETIC ACTION. ... FORM OF MOLECULE ACTIVE IN NERVE FIBERS IS CATION. ... CATION ... COMBINES WITH SOME RECEPTOR IN MEMBRANE TO PREVENT GENERATION OF ACTION POTENTIAL. /LOCAL ANESTHETICS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 383
... /SUGGESTED/ THAT PROCAINE ... DIMINISHES RELEASE OF ACETYLCHOLINE BY MOTOR-NERVE ENDINGS. /PROCAINE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 385
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?