
Synopsis
Synopsis
0
EU WC
0
KDMF
0
VMF
0
Europe

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
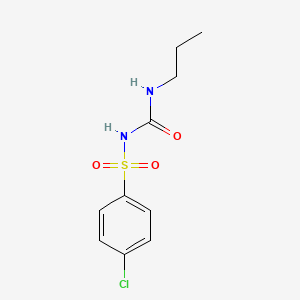

1. Apo-chlorpropamide
2. Clorpropamid
3. Diabinese
4. Glucamide
5. Insogen
6. Meldian
1. 94-20-2
2. Chloropropamide
3. Diabinese
4. Glucamide
5. Meldian
6. Chlorpropamid
7. Diabenese
8. Chlorodiabina
9. Chloronase
10. Diabeneza
11. Diabetoral
12. Adiaben
13. Catanil
14. Diabaril
15. Dynalase
16. Insulase
17. Melitase
18. Diabechlor
19. Diabenal
20. Mellinese
21. Millinese
22. Asucrol
23. Glisema
24. Oradian
25. Diabet-pages
26. Diamel Ex
27. 1-(4-chlorophenylsulfonyl)-3-propylurea
28. 4-chloro-n-(propylcarbamoyl)benzenesulfonamide
29. Clorpropamide
30. Diabexan
31. Prodiaben
32. Chlorpropamidum
33. Clorpropamida
34. 1-(p-chlorobenzenesulfonyl)-3-propylurea
35. 1-(4-chlorophenyl)sulfonyl-3-propylurea
36. 1-propyl-3-(p-chlorobenzenesulfonyl)urea
37. N-(4-chlorophenylsulfonyl)-n'-propylurea
38. N-(p-chlorobenzenesulfonyl)-n'-propylurea
39. N-propyl-n'-(p-chlorobenzenesulfonyl)urea
40. 4-chloro-n-[(propylamino)carbonyl]benzenesulfonamide
41. 1-(p-chlorophenylsulfonyl)-3-propylurea
42. 1-p-chlorophenyl-3-(propylsulfonyl)urea
43. Nci-c01752
44. 4-chloro-n-((propylamino)carbonyl)benzenesulfonamide
45. N-propyl-n'-p-chlorphenylsulfonylcarbamide
46. 1-((p-chlorophenyl)sulfonyl)-3-propylurea
47. N-propyl-n'-p-chlorophenylsulfonylcarbamide
48. 1-[(4-chlorobenzene)sulfonyl]-3-propylurea
49. P 607
50. Nsc 44634
51. Benzenesulfonamide, 4-chloro-n-((propylamino)carbonyl)-
52. U-9818
53. 4-chloro-4-((propylamino)carbonyl)benzenesulfonamide
54. Benzenesulfonamide, 4-chloro-n-[(propylamino)carbonyl]-
55. Clorpropamid
56. Mfcd00079004
57. Nsc-44634
58. Wtm2c3il2x
59. Chembl498
60. Nsc-626720
61. Mls000028395
62. Chebi:3650
63. Bioglumin
64. Insogen
65. Urea, 1-((p-chlorophenyl)sulfonyl)-3-propyl-
66. Nsc626720
67. Cas-94-20-2
68. Ncgc00015216-11
69. Chlorporpamide
70. Smr000058364
71. Clorpropamide [dcit]
72. Dsstox_cid_322
73. Chlorpropamide, Alpha-form
74. Clorpropamide [italian]
75. Urea, 1-[(p-chlorophenyl)sulfonyl]-3-propyl-
76. Chlorpropamide, Epsilon-form
77. Dsstox_rid_75512
78. Dsstox_gsid_20322
79. Chlorpropamide, Epsilon`-form
80. Chlorpropamidum [inn-latin]
81. Clorpropamida [inn-spanish]
82. Diabinese (tn)
83. Ccris 155
84. 1-(p-chlorobenzensulfonyl)-3-propylurea
85. Hsdb 2051
86. 4-chloro-n-(propylaminocarbonyl)benzenesulfonamide
87. Sr-01000000060
88. 4-chloro-n-((propylaminocarbonyl)benzenesulfonamide
89. Einecs 202-314-5
90. Unii-wtm2c3il2x
91. U-3818
92. Nsc 626720
93. Brn 2218363
94. Urea, 1-((p-chloropenyl)sulfonyl)-3-propyl-
95. Prestwick_684
96. Chlorpropamide [usp:inn:ban:jan]
97. Chlorpropamide B.p.
98. Chlorpropamide-[d4]
99. Spectrum_000144
100. 1-(4-chlorobenzenesulfonyl)-3-propylurea
101. 1-(4-chlorophenyl)sulfonyl-3-propyl-urea
102. Opera_id_359
103. Adenylosuccinicacid
104. Prestwick0_000323
105. Prestwick1_000323
106. Prestwick2_000323
107. Prestwick3_000323
108. Spectrum2_000089
109. Spectrum3_000347
110. Spectrum4_000284
111. Spectrum5_000719
112. Wln: Gr Dswmvm3
113. Lopac-c-1290
114. Chlorpropamide, Delta-form
115. 1-(4-chloro-benzenesulfonyl)-3-n-propyl-urea
116. C 1290
117. Chlorpropamide [mi]
118. Chlorpropamide [inn]
119. Chlorpropamide [jan]
120. Lopac0_000229
121. Schembl23947
122. Bspbio_000325
123. Bspbio_002013
124. Chlorpropamide [hsdb]
125. Kbiogr_000808
126. Kbiogr_002273
127. Kbioss_000624
128. Kbioss_002274
129. Mls001148665
130. Chlorpropamide [vandf]
131. Divk1c_000513
132. Spectrum1500185
133. Spbio_000018
134. Spbio_002246
135. Chlorpropamide [mart.]
136. Bpbio1_000359
137. Gtpl6801
138. Chlorpropamide [usp-rs]
139. Chlorpropamide [who-dd]
140. Dtxsid9020322
141. Hms501j15
142. Kbio1_000513
143. Kbio2_000624
144. Kbio2_002273
145. Kbio2_003192
146. Kbio2_004841
147. Kbio2_005760
148. Kbio2_007409
149. Kbio3_001233
150. Kbio3_002753
151. Chlorpropamide (jp17/usp/inn)
152. Cmap_000007
153. Ninds_000513
154. Hms1569a07
155. Hms1920m05
156. Hms2091e08
157. Hms2096a07
158. Hms2233l19
159. Hms3259a17
160. Hms3260n19
161. Hms3373d09
162. Hms3428c03
163. Hms3652l03
164. Hms3713a07
165. Pharmakon1600-01500185
166. Bcp09162
167. Hy-b1429
168. Nsc44634
169. Zinc1530599
170. Chlorpropamide [ep Impurity]
171. Chlorpropamide [orange Book]
172. Tox21_110102
173. Tox21_201391
174. Tox21_302789
175. Tox21_500229
176. Ac8695
177. Bdbm50344965
178. Ccg-38905
179. Nsc756690
180. Nsc813219
181. S4166
182. Stk857458
183. Chlorpropamide [usp Monograph]
184. Akos001482739
185. Tox21_110102_1
186. Cs-4917
187. Db00672
188. Ks-5316
189. Lp00229
190. Nc00503
191. Nsc-756690
192. Nsc-813219
193. Sdccgsbi-0050217.p005
194. Idi1_000513
195. Mrf-0000539
196. Ncgc00015216-01
197. Ncgc00015216-02
198. Ncgc00015216-03
199. Ncgc00015216-04
200. Ncgc00015216-05
201. Ncgc00015216-06
202. Ncgc00015216-07
203. Ncgc00015216-08
204. Ncgc00015216-09
205. Ncgc00015216-10
206. Ncgc00015216-12
207. Ncgc00015216-13
208. Ncgc00015216-14
209. Ncgc00015216-17
210. Ncgc00015216-18
211. Ncgc00015216-23
212. Ncgc00021451-03
213. Ncgc00021451-04
214. Ncgc00021451-05
215. Ncgc00021451-06
216. Ncgc00021451-07
217. Ncgc00021451-08
218. Ncgc00256414-01
219. Ncgc00258942-01
220. Ncgc00260914-01
221. Sy052508
222. Sbi-0050217.p004
223. Ab00051944
224. C1220
225. Chlorpropamide, Analytical Standard, >=97%
226. Eu-0100229
227. P-607
228. Sw196839-3
229. A16447
230. D00271
231. Urea, 1-propyl-3-(p-chloro-benzenesulfonyl)-
232. Ab00051944_16
233. Ab00051944_17
234. Q1075324
235. Sr-01000000060-2
236. Sr-01000000060-4
237. Sr-01000000060-6
238. W-100205
239. 4-chloro-n-[(propylamino)-carbonyl]benzenesulfonamide
240. Brd-k97746869-001-05-6
241. Brd-k97746869-001-15-5
242. 1-chloro-4-(([(propylamino)carbonyl]amino)sulfonyl)benzene #
243. Chlorpropamide, European Pharmacopoeia (ep) Reference Standard
244. Chlorpropamide, United States Pharmacopeia (usp) Reference Standard
245. Chlorpropamide, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 276.74 g/mol |
|---|---|
| Molecular Formula | C10H13ClN2O3S |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 276.0335411 g/mol |
| Monoisotopic Mass | 276.0335411 g/mol |
| Topological Polar Surface Area | 83.6 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 345 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Chlorpropamide |
| PubMed Health | Chlorpropamide (By mouth) |
| Drug Classes | Hypoglycemic |
| Drug Label | Chlorpropamide is an oral blood-glucose-lowering drug of the sulfonylurea class. Chlorpropamide, USP is a white, crystalline powder, that has a slight odor. It is practically insoluble in water, but is soluble in alcohol. Chemically, it is 4-chloro-N... |
| Active Ingredient | Chlorpropamide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 100mg |
| Market Status | Prescription |
| Company | Ani Pharms; Mylan |
| 2 of 4 | |
|---|---|
| Drug Name | Diabinese |
| PubMed Health | Chlorpropamide (By mouth) |
| Drug Classes | Hypoglycemic |
| Active Ingredient | Chlorpropamide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 100mg |
| Market Status | Prescription |
| Company | Pfizer |
| 3 of 4 | |
|---|---|
| Drug Name | Chlorpropamide |
| PubMed Health | Chlorpropamide (By mouth) |
| Drug Classes | Hypoglycemic |
| Drug Label | Chlorpropamide is an oral blood-glucose-lowering drug of the sulfonylurea class. Chlorpropamide, USP is a white, crystalline powder, that has a slight odor. It is practically insoluble in water, but is soluble in alcohol. Chemically, it is 4-chloro-N... |
| Active Ingredient | Chlorpropamide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 100mg |
| Market Status | Prescription |
| Company | Ani Pharms; Mylan |
| 4 of 4 | |
|---|---|
| Drug Name | Diabinese |
| PubMed Health | Chlorpropamide (By mouth) |
| Drug Classes | Hypoglycemic |
| Active Ingredient | Chlorpropamide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 100mg |
| Market Status | Prescription |
| Company | Pfizer |
Hypoglycemic Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Sulfonylureas are used to control hyperglycemia in NIDDM pt who cannot achieve appropriate control with changes in diet alone. /Sulfonylurea/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1509
...EFFECTIVE IN MATURITY-ONSET DIABETIC PT IN WHOM PANCREAS RETAINS CAPACITY TO SECRETE INSULIN. /SULFONYLUREAS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1520
MEDICATION (VET): IN DIABETES MELLITUS IN DOGS.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 110
For more Therapeutic Uses (Complete) data for CHLORPROPAMIDE (8 total), please visit the HSDB record page.
...STUDIES...INDICATE INCR INCIDENCE OF...DIFFICULTIES IN PT TAKING ORAL HYPOGLYCEMIC DRUG. ...VENTRICULAR TACHYCARDIA &...FIBRILLATION WERE NOTED...USUALLY DURING EARLY STAGES OF MYOCARDIAL INFARCTION... /SULFONYLUREA/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1522
SULFONYLUREAS SHOULD BE ADMINISTERED WITH CAUTION TO PT WITH EITHER RENAL OR HEPATIC INSUFFICIENCY /SULFONYLUREAS/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1509
VET: AVOID USE IN PREGNANT ANIMALS.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 110
VET: /SULFONYLUREA SUBSTANCES/...HAVE BEEN OF LITTLE VALUE IN CANINE DIABETES TREATMENT. ONLY MILDEST CASES HAVE RESPONDED AT ALL. /HYPOGLYCEMIC SULFONYLUREA/
Jones, L.M., et al. Veterinary Pharmacology & Therapeutics. 4th ed. Ames: Iowa State University Press, 1977., p. 704
For more Drug Warnings (Complete) data for CHLORPROPAMIDE (18 total), please visit the HSDB record page.
For treatment of NIDDM in conjunction with diet and exercise.
Chlorpropamide, a second-generation sulfonylurea antidiabetic agent, is used with diet to lower blood glucose levels in patients with diabetes mellitus type II. Chlorpropamide is twice as potent as the related second-generation agent glipizide.
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BB - Sulfonylureas
A10BB02 - Chlorpropamide
Absorption
Readily absorbed from the GI tract. Peak plasma concentrations occur within 2-4 hours and the onset of action occurs within one hour. The maximal effect of chlorpropamide is seen 3-6 hours following oral administration.
Route of Elimination
80-90% of a single oral dose is excreted in the urine as unchaged drug and metabolites within 96 hours.
...EFFECTIVELY ABSORBED FROM GI TRACT ...
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1508
Excreted (percentage)...60 /from table/
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 722
... 20% excreted unchanged; ... /from table/
Young, L.Y., M.A. Koda-Kimble (eds.). Applied Therapeutics. The Clinical Use of Drugs. 6th ed. Vancouver, WA., Applied Therapeutics, Inc. 1995., p. 48-37
Chlorpropamide is readily absorbed from the GI tract following oral administration. Following oral administration of a single dose, the drug is detectable in plasma within 1 hour and peak plasma chlorpropamide concentrations occur within 2-4 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2732
For more Absorption, Distribution and Excretion (Complete) data for CHLORPROPAMIDE (6 total), please visit the HSDB record page.
Up to 80% of dose is metabolized likely through the liver to to 2-hydroxylchlorpropamide (2-OH CPA), p-chlorobenzenesulfonylurea (CBSU), 3-hydroxylchlorpropamide (3-OH CPA), and p-chlorobenzenesulfonamide (CBSA); CBSA may be produced by decomposition in urine. It is unknown whether chlorpropamide metabolites exert hypoglycemic effects.
...METABOLISM OF CHLORPROPAMIDE IS INCOMPLETE, AND ABOUT 20% OF THE DRUG IS EXCRETED UNCHANGED
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1509
...SOME HYDROLYTIC BREAKDOWN OF ACTUAL UREA MOIETY HAS BEEN DETECTED, RESULTING IN FORMATION OF SULFONAMIDE DERIV... RECENT EVIDENCE SUGGESTS THIS...TO BE ARTIFACTUAL & NOT A GENUINE METABOLITE...
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 145
FOLLOWING PER ORAL ADMIN TO MAN OF TRITIATED CHLORPROPAMIDE...80% OF DOSE WAS EXCRETED...DURING 7-DAY PERIOD. METABOLITES...WERE P-CHLOROBENZENESULFONAMIDE...[(P-CHLOROPHENYL)SULFONYL]UREA... 1-[(P-CHLOROPHENYL)SULFONYL]-3-(2-HYDROXYPROPYL)UREA. ..&.1-[(P-CHLOROPHENYL)SULFONYL]-3-(3-HYDROXYPROPYL)UREA...
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 558
...DIABETIC PT WERE ADMIN.../CHLORPROPAMIDE/... AT...250-500 MG...PRODUCTS EXCRETED...INCL.../(P-CHLOROPHENYL)SULFONYLUREA/ (21%)... /P-CHLOROBENZENESULFONAMIDE/ (2%), 2-HYDROXYCHLORPROPAMIDE (55%), & 3-HYDROXYCHLORPROPAMIDE (2%).
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 305
For more Metabolism/Metabolites (Complete) data for CHLORPROPAMIDE (6 total), please visit the HSDB record page.
Chlorpropamide has known human metabolites that include 2-hydroxy-chlorpropamide, 3-hydroxy-chlorpropamide, and p-Chlorobenzene sulfonylurea.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Approximately 36 hours with interindividual variation ranging from 25-60 hours. Duration of effect persists for at least 24 hours.
Chlorpropamid has a long half-life (24 to 48 hours).
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1509
Half-life...24-48 /hours/ /from table/
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 722
Sulfonylureas such as chlorpropamide bind to ATP-sensitive potassium channels on the pancreatic cell surface, reducing potassium conductance and causing depolarization of the membrane. Depolarization stimulates calcium ion influx through voltage-sensitive calcium channels, raising intracellular concentrations of calcium ions, which induces the secretion, or exocytosis, of insulin.
...ACTION OF SULFONYLUREAS APPEARS TO BE STIMULATION OF RELEASE OF INSULIN FROM BETA CELLS. ...TO BE EFFECTIVE, PT MUST HAVE SOME FUNCTIONAL ISLET CELLS... /HYPOGLYCEMIC SULFONYLUREAS/
Jones, L.M., et al. Veterinary Pharmacology & Therapeutics. 4th ed. Ames: Iowa State University Press, 1977., p. 704
Sulfonylureas cause hypoglycemia by stimulating insulin release from pancreatic beta cells. Their effects in the treatment of diabetes ... are more complex. /Sulfonylureas/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1507
Sulfonylureas are now...thought to act by a number of different mechanisms. 1. ...produce a depolarization of the pancreatic islet beta cell membrane potassium ion permeability. This results in a release of preformed insulin into the circulation and occurs mostly in non-insulin dependent diabetics. 2. ...reduce basal glucose output from the liver... 3. increase insulin receptor binding... 4. ...increasing intracellular levels of AMP... 5. increase insulin secretion by suppressing the release of glucagon and somatostatin from alpha and delta pancreatic cells. /Sulfonylureas/
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 723
Sulfonylureas lower blood glucose in NIDDM by directly stimulating the acute release of insulin from functioning beta cells of pancreatic islet tissue by an unknown process that involves a sulfonylurea receptor on the beta cell. Sulfonylureas inhibit the ATP potassium channels on the beta cell membrane and potassium efflux, which results in depolarization and calcium influx, calcium-calmodulin binding, kinase activation, and release of insulin containing granules by exocytosis, an effect similar to that of glucose. Insulin is a hormone that lowers blood glucose and controls the storage and metabolism of carbohydrates, proteins, and fats. Therefore, sulfonylureas are effective only in patients whose pancreata are capable of producing insulin. /Sulfonylurea antidiabetic agents/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 19th ed. Volume I.Micromedex, Inc. Englewood, CO., 1999. Content Prepared by the U.S. Pharmacopieal Convention, Inc., p. 284

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
71
PharmaCompass offers a list of Chlorpropamide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Chlorpropamide manufacturer or Chlorpropamide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Chlorpropamide manufacturer or Chlorpropamide supplier.
PharmaCompass also assists you with knowing the Chlorpropamide API Price utilized in the formulation of products. Chlorpropamide API Price is not always fixed or binding as the Chlorpropamide Price is obtained through a variety of data sources. The Chlorpropamide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Chlorpropamide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Chlorpropamide, including repackagers and relabelers. The FDA regulates Chlorpropamide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Chlorpropamide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Chlorpropamide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Chlorpropamide supplier is an individual or a company that provides Chlorpropamide active pharmaceutical ingredient (API) or Chlorpropamide finished formulations upon request. The Chlorpropamide suppliers may include Chlorpropamide API manufacturers, exporters, distributors and traders.
click here to find a list of Chlorpropamide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Chlorpropamide DMF (Drug Master File) is a document detailing the whole manufacturing process of Chlorpropamide active pharmaceutical ingredient (API) in detail. Different forms of Chlorpropamide DMFs exist exist since differing nations have different regulations, such as Chlorpropamide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Chlorpropamide DMF submitted to regulatory agencies in the US is known as a USDMF. Chlorpropamide USDMF includes data on Chlorpropamide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Chlorpropamide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Chlorpropamide suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Chlorpropamide Drug Master File in Japan (Chlorpropamide JDMF) empowers Chlorpropamide API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Chlorpropamide JDMF during the approval evaluation for pharmaceutical products. At the time of Chlorpropamide JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Chlorpropamide suppliers with JDMF on PharmaCompass.
A Chlorpropamide CEP of the European Pharmacopoeia monograph is often referred to as a Chlorpropamide Certificate of Suitability (COS). The purpose of a Chlorpropamide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Chlorpropamide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Chlorpropamide to their clients by showing that a Chlorpropamide CEP has been issued for it. The manufacturer submits a Chlorpropamide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Chlorpropamide CEP holder for the record. Additionally, the data presented in the Chlorpropamide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Chlorpropamide DMF.
A Chlorpropamide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Chlorpropamide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Chlorpropamide suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Chlorpropamide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Chlorpropamide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Chlorpropamide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Chlorpropamide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Chlorpropamide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Chlorpropamide suppliers with NDC on PharmaCompass.
Chlorpropamide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Chlorpropamide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Chlorpropamide GMP manufacturer or Chlorpropamide GMP API supplier for your needs.
A Chlorpropamide CoA (Certificate of Analysis) is a formal document that attests to Chlorpropamide's compliance with Chlorpropamide specifications and serves as a tool for batch-level quality control.
Chlorpropamide CoA mostly includes findings from lab analyses of a specific batch. For each Chlorpropamide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Chlorpropamide may be tested according to a variety of international standards, such as European Pharmacopoeia (Chlorpropamide EP), Chlorpropamide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Chlorpropamide USP).
