
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
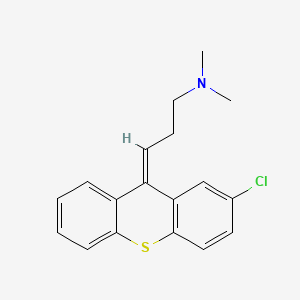

1. Chlorprotixen
2. Taractan
1. 113-59-7
2. Taractan
3. Truxal
4. Clorprotixeno
5. Chlorprothixen
6. Chlorprotixen
7. Chlorprotixene
8. Chlothixen
9. Iaractan
10. Rentovet
11. Traquilan
12. Vetacalm
13. Trictal
14. Truxil
15. Chlorprothixenum
16. Paxyl
17. Tardan
18. N-714
19. (z)-chlorprothixene
20. Ro 4-0403
21. Mk 184
22. N 714c
23. Truxaletten
24. Cis-2-chloro-9-(3-dimethylaminopropylidene)thioxanthene
25. Ro-4-0403
26. (z)-2-chloro-9-(omega-dimethylaminopropylidene)thioxanthene
27. (z)-3-(2-chloro-9h-thioxanthen-9-ylidene)-n,n-dimethylpropan-1-amine
28. N 714
29. Nsc-18720
30. Chembl908
31. 9s7od60ewp
32. (3z)-3-(2-chloro-9h-thioxanthen-9-ylidene)-n,n-dimethylpropan-1-amine
33. Tactaran
34. Chebi:50931
35. (3z)-3-(2-chlorothioxanthen-9-ylidene)-n,n-dimethylpropan-1-amine
36. Nsc18720
37. Ro-40403
38. Clorprotisene
39. N-714ro 4-0403
40. Clorprotisene [dcit]
41. Cloxan
42. 1-propanamine, 3-(2-chloro-9h-thioxanthen-9-ylidene)-n,n-dimethyl-, (z)-
43. {3-[(9z)-2-chloro-9h-thioxanthen-9-ylidene]propyl}dimethylamine
44. .alpha.-chlorprothixene
45. Clorprotixeno [inn-spanish]
46. Taractan (tn)
47. Chlorprothixenum [inn-latin]
48. Unii-9s7od60ewp
49. Hsdb 2808
50. Chlorprothixene (jan/usan/inn)
51. Einecs 204-032-8
52. Chlorprothixene,(s)
53. Nsc 18720
54. Nsc56379
55. Chlorprothixene [usan:usp:inn:ban:jan]
56. Prestwick2_000348
57. (z)-2-chloro-n,n-dimethylthioxanthene-delta(sup 9,gamma)-propylamine
58. Chlorprothixene [mi]
59. Schembl94235
60. Schembl94236
61. Chlorprothixene [inn]
62. Chlorprothixene [jan]
63. Mls000768404
64. Chlorprothixene [hsdb]
65. Chlorprothixene [usan]
66. Chlorprothixene [vandf]
67. 2-chloro-9-[3-(dimethylamino)propylidene]thioxanthene
68. Zinc1137
69. Chlorprothixene [mart.]
70. Chlorprothixene [who-dd]
71. Dtxsid4022810
72. Gtpl11976
73. 2-chloro-n, .gamma.-propylamine
74. 2-chloro-9-[.omega.-(dimethylamino)propylidene]thioxanthene
75. Hms2788n19
76. Hms3884c10
77. {3-[2-chloro-thioxanthen-(9z)-ylidene]-propyl}-dimethyl-amine
78. 1-propanamine,n-dimethyl-, (z)-
79. Hy-b0274
80. Bdbm50240514
81. Ccg-36988
82. Chlorprothixene [orange Book]
83. Mfcd00869180
84. Pdsp1_001124
85. Pdsp2_001108
86. S1771
87. Stk545163
88. 1-propanamine, 3-(2-chloro-9h-thioxanthen-9-ylidene)-n,n-dimethyl-, (3z)-
89. Akos005065593
90. Db01239
91. Ks-5102
92. Ncgc00166133-01
93. Smr000431796
94. (z)-2-chloro-n,(sup.gamma.)-propylamine
95. A8398
96. C3505
97. Chlorprothixene 100 Microg/ml In Acetonitrile
98. C07953
99. D00790
100. Wln: T C666 Bs Iyj Fg Iu3n1 & 1
101. 113c597
102. J-002997
103. Brd-k36207157-001-09-6
104. Brd-k81091703-003-01-0
105. Q63395672
106. Thioxanthene-.delta.(sup 9), 2-chloro-n,n-dimethyl-
107. (z)-2-chloro-9-(3-dimethylaminopropylidene)thioxanthene
108. Thioxanthene-.delta.9, 2-chloro-n,n-dimethyl-, (z)-
109. (.alpha.-2-chloro-9-.omega.-dimethylamino-propylamine)thioxanthene
110. (3z)-3-(2-chlorothioxanthen-9-ylidene)-n,n-dimethyl-propan-1-amine
111. Thioxanthene-delta(sup 9),gamma-propylamine, 2-chloro-n,n-dimethyl-, (z)-
112. Thioxanthene-delta9,gamma-propylamine, 2-chloro-n,n-dimethyl-, (z)-
113. (z)-2-chloro-n,n-dimethylthioxanthene-.delta.(sup 9,.gamma.)-propylamine
| Molecular Weight | 315.9 g/mol |
|---|---|
| Molecular Formula | C18H18ClNS |
| XLogP3 | 5.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 315.0848484 g/mol |
| Monoisotopic Mass | 315.0848484 g/mol |
| Topological Polar Surface Area | 28.5 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 381 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antipsychotic Agents; Dopamine Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
IT MAY BE OF VALUE IN THE SYMPTOMATIC TREATMENT OF AGITATED STATES ASSOCIATED WITH NEUROSES, DEPRESSION OR SCHIZOPHRENIA. DRUG APPEARS TO BE MORE EFFECTIVE IN THE MANAGEMENT OF ACUTE SCHIZOPHRENIA THAN OF CHRONIC SCHIZOPHRENIA.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 28:16
...IT HAS BEEN USED IN THE TREATMENT OF...PSYCHOTIC & SEVERE NEUROTIC CONDITIONS IN WHICH ANXIETY, AGITATION, AND TENSION PREDOMINATE.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1023
.../IT/ MAY BE USED TO POTENTIATE CENTRAL NERVOUS SYSTEM DEPRESSANTS & CONCOMITANTLY WITH ANTICONVULSANTS &/OR ELECTROSHOCK TREATMENT.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 28:16
Indicated for management of primary and secondary symptoms of psychotic disorders. /Thioxanthenes; Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 2844
CHLORPROTHIXENE IS CONTRAINDICATED IN PATIENTS WHO ARE HYPERSENSITIVE TO THE DRUG; THE POSSIBILITY OF CROSS-SENSITIVITY TO PHENOTHIAZINES & TO THIOTHIXENE MUST BE BORNE IN MIND.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 28:16
CHLORPROTHIXENE IS CONTRAINDICATED IN PATIENTS WITH CIRCULATORY COLLAPSE & IN THOSE WITH CONGESTIVE FAILURE, CARDIAC DECOMPENSATION, CORONARY ARTERY, OR CEREBRAL VASCULAR DISORDERS.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 28:16
CHLORPROTHIXENE IS CONTRAINDICATED IN COMATOSE STATES, PARTICULARLY THOSE INDUCED BY CNS DEPRESSANT DRUGS.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 28:16
WHEN CHLORPROTHIXENE IS USED CONCOMITANTLY WITH OTHER CNS DEPRESSANTS, CAUTION MUST BE OBSERVED TO AVOID OVERDOSAGE...
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 28:16
For more Drug Warnings (Complete) data for CHLORPROTHIXENE (27 total), please visit the HSDB record page.
4(?). 4= VERY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 50-500 MG/KG; BETWEEN 1 TEASPOON AND 1 OUNCE FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-221
For treatment of psychotic disorders (e.g. schizophrenia) and of acute mania occuring as part of bipolar disorders.
Chlorprothixene is a typical antipsychotic drug of the thioxanthine class. It has a low antipsychotic potency (half to 2/3 of chlorpromazine). Chlorprothixene has not thoroughly demonstrated an antidepressant or analgesic effect but it has demonstrated antiemetic effects. It is used in the treatment of nervous, mental, and emotional conditions. Improvement in such conditions is thought to result from the effect of the medicine on nerve pathways in specific areas of the brain. Chlorprothixene has a similar side effect profile to chlorpromazine, though allergic side effects and liver damage are less frequent.
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AF - Thioxanthene derivatives
N05AF03 - Chlorprothixene
Absorption
Incomplete bioavailability.
.../IT/ IS PARTIALLY ABSORBED FROM THE GI TRACT. FOLLOWING IM ADMIN, THE DRUG EXERTS ITS EFFECTS WITHIN 10-30 MINUTES. IT IS METABOLIZED, PRESUMABLY IN THE LIVER...FREE CHLORPROTHIXENE & ITS SULFOXIDE METABOLITE ARE EXCRETED IN THE URINE & FECES.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 28:16
TRICYCLIC AGENT, CHLORPROTHIXENE, IS...EXTENSIVELY DISTRIBUTED IN BODY & HAS OVERALL DISTRIBUTION VOL IN MAN APPROACHING 1000 L...
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 4: A Review of the Literature Published during 1974 and 1975. London: The Chemical Society, 1977., p. 11
AFTER IV ADMIN PHARMACOKINETICS FOLLOWED 2 COMPARTMENT MODEL. ...TOTAL VOL OF DISTRIBUTION WAS VERY LARGE. AFTER ORAL ADMIN (15 MG) BIOAVAILABILITY WAS POOR, ALTHOUGH ABSORPTION OF CMPD FROM THE GUT APPEARED TO BE GOOD.
PMID:1140253 RAAFLAUB J; EXPERIENTIA 31 (5): 557 (1975)
METABOLISM OCCURRED MORE RAPIDLY WITH INCR AGE FROM 3-24 WK IN RATS. LEVELS OF CHLORPROTHIXENE, N-DEMETHYLCHLORPROTHIXENE & TOTAL AMINES IN BRAIN, LIVER, KIDNEYS & LUNG OF 3-WK OLD RATS WERE ABOUT TWICE THOSE IN 6-WK OLD RATS. AMT IN ORGANS WERE HIGHER IN FEMALES THAN IN MALES.
DELL ET AL; ARZNEIM-FORSCH 26 (6): 1098 (1976)
After 1 hour intravenous chlorprothixene infusion, the maximum serum concentration of chlorprothixene was 430 ng/ml, which subsequently decreased with a terminal elimination half-life of 25.8 hours. The total serum clearance and the apparent volume of distribution at steady state were 867 ml/min and 1035 l, respectively. ... Chlorprothixene bioavailability relative to the oral soution was 56.45 with the coated tablet and 67.7% with the suspension. All pharmacokinetic parameterws showed wide inter-subject varioations.
Bagli M et al; Arzneim Forsch 46 (3): 247-50 (1996)
Hepatic
UNCHANGED CHLORPROTHIXENE, THE SULFOXIDE DERIVATIVE & N-DEMETHYL SULFOXIDE HAVE BEEN IDENTIFIED IN THE URINE OF TREATED DOGS & RATS. HYDROXYLATION & GLUCURONIDATION ALSO OCCUR IN DOGS, BUT IDENTIFICATION OF THOSE BIOTRANSFORMATION PRODUCTS HAS NOT BEEN ACCOMPLISHED.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 166
YIELDS DEMETHYLCHLORPROTHIXENE IN RAT. /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-46
FOLLOWING ORAL ADMIN TO DOG & PSYCHIATRIC PT SEVERAL 3-, 7-, 4-, 6-, & 8-HYDROXYLATED METABOLITES FOUND IN URINE & FECES. HYDROXYLATION WAS FOLLOWED BY CONJUGATION WITH GLUCURONIC &/OR SULFURIC ACID FOR EXCRETION.
WIEST ET AL; DEV NEUROSCI (AMSTERDAM) 7, ISS PHENOTHIAZINES STRUCT RELAT DRUGS: BASIC CLIN STUD 177 (1980)
DRUG ADMIN TO RATS BY GAVAGE AT 100 MG/KG WAS METABOLIZED MORE RAPIDLY WITH INCR AGE FROM 3-24 WK.
DELL ET AL; ARZNEIM-FORSCH 26 (6): 1098 (1976)
8 to 12 hours
AFTER IV ADMIN PHARMACOKINETICS FOLLOWED 2 COMPARTMENT MODEL. T/2 IN SECOND PHASE OF ELIMINATION WAS 5-12 HR...
PMID:1140253 RAAFLAUB J; EXPERIENTIA 31 (5): 557 (1975)
TRICYCLIC AGENT, CHLORPROTHIXENE, ...HAS...BIOLOGICAL T/2 OF 8-12 HR.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 4: A Review of the Literature Published during 1974 and 1975. London: The Chemical Society, 1977., p. 11
Chlorprothixene blocks postsynaptic mesolimbic dopaminergic D1 and D2 receptors in the brain; depresses the release of hypothalamic and hypophyseal hormones and is believed to depress the reticular activating system thus affecting basal metabolism, body temperature, wakefulness, vasomotor tone, and emesis.
...IT CAN BE EXPECTED TO DEPRESS THE CNS AT THE SUBCORTICAL LEVEL OF THE BRAIN, THE MIDBRAIN, & THE BRAIN STEM RETICULAR FORMATION.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 28:16
.../IT/ IS MORE ACTIVE THAN CHLORPROMAZINE IN INHIBITING POSTURAL REFLEXES & MOTOR COORDINATION & LESS ACTIVE IN ANTIHISTAMINIC EFFECTS. CHLORPROTHIXENE POSSESSES SEDATIVE, ADRENOLYTIC, HYPOTHERMIC, ANTICHOLINERGIC, & ANTIEMETIC PROPERTIES.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 28:16
Thioxanthenes are thought to benefit psychotic conditions by blocking postsynaptic dopamine receptors in the brain. They also produce an alpha-adrenergic blocking effect and depress the release of most hypothalamic and hypophyseal hormones. However, the concentration of prolactin is increased due to blockade of prolactin inhibitory factor (PIF), which inhibits the release of prolactin from the pituitary gland. /Thioxanthenes/
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 2844
Chlorprothixene also inhibits the medullary chemoreceptor trigger zone to produce an antiemetic effect, and is also thought to cause an indirect reduction of stimuli to the brain stem reticular system to produce a sedative effect.
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 2844
Global Sales Information

Market Place

ABOUT THIS PAGE
40
PharmaCompass offers a list of Chlorprothixene API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Chlorprothixene manufacturer or Chlorprothixene supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Chlorprothixene manufacturer or Chlorprothixene supplier.
PharmaCompass also assists you with knowing the Chlorprothixene API Price utilized in the formulation of products. Chlorprothixene API Price is not always fixed or binding as the Chlorprothixene Price is obtained through a variety of data sources. The Chlorprothixene Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Chlorprothixene manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Chlorprothixene, including repackagers and relabelers. The FDA regulates Chlorprothixene manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Chlorprothixene API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Chlorprothixene supplier is an individual or a company that provides Chlorprothixene active pharmaceutical ingredient (API) or Chlorprothixene finished formulations upon request. The Chlorprothixene suppliers may include Chlorprothixene API manufacturers, exporters, distributors and traders.
Chlorprothixene Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Chlorprothixene GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Chlorprothixene GMP manufacturer or Chlorprothixene GMP API supplier for your needs.
A Chlorprothixene CoA (Certificate of Analysis) is a formal document that attests to Chlorprothixene's compliance with Chlorprothixene specifications and serves as a tool for batch-level quality control.
Chlorprothixene CoA mostly includes findings from lab analyses of a specific batch. For each Chlorprothixene CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Chlorprothixene may be tested according to a variety of international standards, such as European Pharmacopoeia (Chlorprothixene EP), Chlorprothixene JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Chlorprothixene USP).
