
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
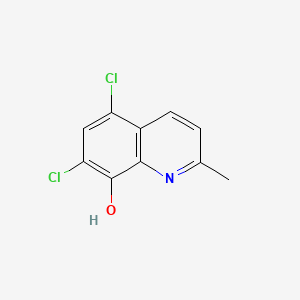

1. 5,7 Dichloro 2 Methyl 8 Quinolinol
2. 5,7-dichloro-2-methyl-8-quinolinol
3. Afungil
4. Chlorchinaldine
5. Chlorchinaldol
6. Chloroquinaldol
7. Sterosan
1. 72-80-0
2. 5,7-dichloro-8-hydroxyquinaldine
3. 5,7-dichloro-2-methylquinolin-8-ol
4. 5,7-dichloro-8-hydroxy-2-methylquinoline
5. Chloroquinaldol
6. 5,7-dichloro-2-methyl-8-quinolinol
7. Clorquinaldol
8. Sterosan
9. 5,7-dichloro-8-quinaldinol
10. Siasteran
11. Siosteran
12. Steroxin
13. Siogene
14. Chlorquinaldolum
15. Gyno-sterosan
16. 5,7-dichloro-2-methyl-8-hydroxyquinoline
17. 8-quinolinol, 5,7-dichloro-2-methyl-
18. Chloquinan
19. Hydroxydichloroquinaldine
20. Siogen
21. 5,7-dichloro-2-methyl-quinolin-8-ol
22. D6vhc87lls
23. Nsc-755830
24. Mls002695929
25. Chlorchinaldol
26. Chebi:74500
27. Chlorchinaldolum
28. Chlorquinaldol (inn)
29. Ncgc00095795-04
30. Clorchinaldolo
31. Smr001549973
32. 2-methyl-5,7-dichloro-8-hydroxyquinoline
33. Chlorquinaldol [inn]
34. Dsstox_cid_28924
35. Dsstox_rid_83190
36. Dsstox_gsid_48998
37. Chlorchinaldin
38. Chlorguinaldon
39. Clorchinaldolo [dcit]
40. Chlorquinaldol, Chlorquinaldol (5,7-dichloro-2-methyl-8-quinolinol)
41. Cas-72-80-0
42. Clorquinaldol [inn-spanish]
43. Chlorquinaldolum [inn-latin]
44. Chlorquinaldol [inn:ban:dcf]
45. Einecs 200-789-3
46. Unii-d6vhc87lls
47. Brn 0156683
48. Gynotherax
49. Vagisteran
50. Florabina
51. Saprosan
52. Siogenal
53. Siogeno
54. Siogenon
55. Siosept
56. Sterozan
57. Quesil
58. Mfcd00023984
59. Hydroxydichloroquinaldinol
60. Spectrum2_000524
61. Spectrum3_001092
62. Spectrum4_001263
63. Chlorquinaldol [mi]
64. Cid_6301
65. Oprea1_721210
66. Bspbio_002764
67. Kbiogr_001846
68. Spectrum212151
69. 5-21-03-00346 (beilstein Handbook Reference)
70. Schembl301405
71. Spbio_000507
72. Chlorquinaldol [mart.]
73. Chembl224325
74. Chlorquinaldol [who-dd]
75. Dtxsid3048998
76. Bdbm76302
77. Kbio3_001984
78. 5-bromo 2-isobutoxy Benzonitirle
79. Hms3089a18
80. Hms3264i07
81. Hms3652h09
82. Pharmakon1600-00212151
83. Zinc119403
84. Bcp11865
85. Hy-b1360
86. Tox21_113490
87. Ccg-39580
88. Nsc755830
89. S4192
90. Stl502989
91. Akos000119838
92. Tox21_113490_1
93. Cs-4899
94. Db13306
95. Nsc 755830
96. Ps-7753
97. S10253
98. Ncgc00095795-01
99. Ncgc00095795-02
100. Ncgc00095795-03
101. Ncgc00095795-06
102. Ac-29743
103. Quinolin-8-ol, 5,7-dichloro-2-methyl-
104. Sbi-0207012.p001
105. Db-055679
106. 5,7-bis(chloranyl)-2-methyl-quinolin-8-ol
107. Ft-0623717
108. Sw220230-1
109. A16448
110. D07208
111. 5,7-dichloro-8-hydroxy-2-methylquinoline, 98%
112. Ab00443827_06
113. Ab00443827_07
114. A837624
115. Sr-01000872737
116. Q1645622
117. Sr-01000872737-1
118. W-104478
119. Z118257784
120. 5,7-dichloro-8-hydroxyquinaldine 5,7-dichloro-2-methyl-8-hydroxyquinoline
| Molecular Weight | 228.07 g/mol |
|---|---|
| Molecular Formula | C10H7Cl2NO |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 226.9904692 g/mol |
| Monoisotopic Mass | 226.9904692 g/mol |
| Topological Polar Surface Area | 33.1 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 214 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Chlorquinaldol was used historically as a topical antiseptic agent for skin infections. It maintains use in European countries as a combination vaginal tablet with promestriene for use in the treatment of vaginal infections.
Chlorquinaldol is bacteriocidal in both gram positive and gram negative bacteria. It is more effective in targeting gram positive bacteria, particularly staphylococci.
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
D - Dermatologicals
D08 - Antiseptics and disinfectants
D08A - Antiseptics and disinfectants
D08AH - Quinoline derivatives
D08AH02 - Chlorquinaldol
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AC - Quinoline derivatives
G01AC03 - Chlorquinaldol
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01A - Agents against amoebiasis and other protozoal diseases
P01AA - Hydroxyquinoline derivatives
P01AA04 - Chlorquinaldol
R - Respiratory system
R02 - Throat preparations
R02A - Throat preparations
R02AA - Antiseptics
R02AA11 - Chlorquinaldol
Absorption
There is a high degree of variability in the extent of absorption of topically applied chorquinaldol preparations. It is reported to be between 4.2 and 23.5% of the applied dose. This is quite low compared to orally administered preparations which displayed 67.6% absorption in the same study.
Route of Elimination
Chlorquinadol is primarily excreted in the urine as the sulfate form. About 2% is excreted as the parent drug.
98% of drug is converted to the sulfate form and renally excreted.
The mechanism by which Chlorquinaldol exerts it's bacteriocidal effect is unknown. 8-hydroxyquinolines are known to be bidentate chelators of several metal ions which act as critical enzyme cofactors. However, the addition of exogenous metal ions does not appear to alter the minimum inhibitory concentration for mycobacterium tuberculosis suggesting that the primary mechanism does not rely on chelation.
Global Sales Information

Market Place

ABOUT THIS PAGE
43
PharmaCompass offers a list of Chlorquinaldol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Chlorquinaldol manufacturer or Chlorquinaldol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Chlorquinaldol manufacturer or Chlorquinaldol supplier.
PharmaCompass also assists you with knowing the Chlorquinaldol API Price utilized in the formulation of products. Chlorquinaldol API Price is not always fixed or binding as the Chlorquinaldol Price is obtained through a variety of data sources. The Chlorquinaldol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Chlorquinaldol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Chlorquinaldol, including repackagers and relabelers. The FDA regulates Chlorquinaldol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Chlorquinaldol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Chlorquinaldol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Chlorquinaldol supplier is an individual or a company that provides Chlorquinaldol active pharmaceutical ingredient (API) or Chlorquinaldol finished formulations upon request. The Chlorquinaldol suppliers may include Chlorquinaldol API manufacturers, exporters, distributors and traders.
click here to find a list of Chlorquinaldol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Chlorquinaldol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Chlorquinaldol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Chlorquinaldol GMP manufacturer or Chlorquinaldol GMP API supplier for your needs.
A Chlorquinaldol CoA (Certificate of Analysis) is a formal document that attests to Chlorquinaldol's compliance with Chlorquinaldol specifications and serves as a tool for batch-level quality control.
Chlorquinaldol CoA mostly includes findings from lab analyses of a specific batch. For each Chlorquinaldol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Chlorquinaldol may be tested according to a variety of international standards, such as European Pharmacopoeia (Chlorquinaldol EP), Chlorquinaldol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Chlorquinaldol USP).
