
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
FDF
0
Europe

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
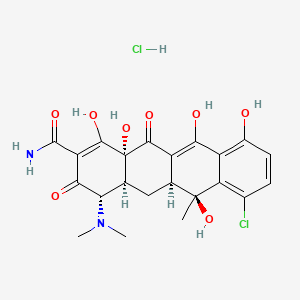

1. 4-epimer Chlortetracycline
2. Aureocyclin
3. Aureomycin
4. Aureomycine
5. Biomycin
6. Bisulfate, Chlortetracycline
7. Calcium Salt Chlortetracycline
8. Chlorotetracycline
9. Chlortetracycline
10. Chlortetracycline Bisulfate
11. Chlortetracycline Monohydrochloride
12. Chlortetracycline Sulfate (1:1)
13. Chlortetracycline Sulfate (2:1)
14. Chlortetracycline, 4 Epimer
15. Chlortetracycline, 4-epimer
16. Chlortetracycline, Calcium Salt
17. Hydrochloride, Chlortetracycline
18. Monohydrochloride, Chlortetracycline
19. Salt Chlortetracycline, Calcium
1. Isphamycin
2. 64-72-2
3. Chlortetracycline Hcl
4. Aureociclina
5. Chlorotetracycline Hydrochloride
6. Aureomycin
7. Aureocycline
8. Aureocarmyl
9. Auxeomycin
10. Clorocipan
11. Fermycin Soluble
12. Aureomycin Monohydrochloride
13. Aureomycin Hydrochloride
14. B-aureo
15. Tetra 5
16. Cltc
17. Biomycin Hydrochloride
18. 7-chlorotetracycline Hydrochloride
19. Biomitsin Hydrochloride
20. Psittacin Hydrochloride
21. Nsc-13252
22. Chlortetracyclinium Chloride
23. Alexomycin
24. Chlortetracycline (hydrochloride)
25. Chlortetracycline, Monohydrochloride
26. 7-chlorotetracycline Monohydrochloride
27. Nsc13252
28. U-6780
29. O1gx33on8r
30. 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide Monohydrochloride
31. 64-72-2 (hcl)
32. Aureomycin (tn)
33. Dsstox_cid_25076
34. Dsstox_rid_80653
35. Dsstox_gsid_45076
36. (4s,4as,5as,6s,12as)-7-chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide Hydrochloride
37. Aurofac 100
38. Aureovit 12c80
39. Pennchlor 64
40. Einecs 200-591-7
41. Clorotetraciclina Cloridrato
42. Clorotetraciclina Cloridrato [italian]
43. Unii-o1gx33on8r
44. Tetracycline, 7-chloro-, Hydrochloride
45. Cltc 100 Mr
46. C22h24cl2n2o8
47. Ai3-50126
48. Sr-05000001588
49. (4s,4as,5as,6s,12as)-7-chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide;hydrochloride
50. Alexomycin [usan]
51. Mfcd26142699
52. Chlortetracycline Hydrochloride [usp:ban]
53. Cas-64-72-2
54. Ncgc00016289-01
55. Schembl3841
56. 2-naphthacenecarboxamide, 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, Monohydrochloride, (4s,4as,5as,6s,12as)-
57. Mls001304009
58. Mls006011591
59. Spectrum1500186
60. Unii-sl2pe81977
61. Chlorotetracycline Hydrochloride 100 Microg/ml In Acetonitrile/water
62. Chlortetracyclini Hydrochloridum
63. Chembl2146063
64. Dtxsid2045076
65. Hms1568m12
66. Hms1920m07
67. Pharmakon1600-01500186
68. Sl2pe81977
69. 3671-08-7
70. Hy-b1327
71. Tox21_110353
72. Tox21_301710
73. Ccg-39737
74. Nsc756691
75. Akos015960769
76. Cs-4853
77. Ds-3304
78. Nsc-756691
79. Ncgc00256236-01
80. 165101-50-8
81. 2-naphthacenecarboxamide, 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, Monohydrochloride (4s-(4alpha,4aalpha,5aalpha,6beta,12aalpha))-
82. Ac-12254
83. Smr000387083
84. Smr004703365
85. Chlortetracycline Hydrochloride (jan/usp)
86. Chlortetracycline Hydrochloride [mi]
87. C3458
88. Chlortetracycline Hydrochloride [jan]
89. Chlortetracycline Hydrochloride [vandf]
90. Chlortetracycline Hydrochloride [mart.]
91. Chlortetracycline Hydrochloride [usp-rs]
92. Chlortetracycline Hydrochloride [who-dd]
93. Chlortetracycline Hydrochloride [who-ip]
94. D02255
95. U 82127
96. U-82127
97. Chlortetracycline Hydrochloride [green Book]
98. Sr-05000001588-2
99. U 82,127
100. U-82,127
101. Chlortetracycline Hydrochloride [ep Monograph]
102. Chlortetracycline Hydrochloride [usp Impurity]
103. Chlortetracycline Hydrochloride [usp Monograph]
104. Chlortetracyclini Hydrochloridum [who-ip Latin]
105. Q27285204
106. Wln: L E6 C666 Bv Fv Cu Guttt&j Dq Eq Gvz Hq In1&1 Mq M1 Og Rq &gh
107. (4s,4as,5as,6s,12ar)-7-chloro-4-(dimethylamino)-1,6,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide;hydrochloride
108. 2-naphthacenecarboxamide, 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, Monohydrochloride (4s-(4.alpha.,4a.alpha.,5a.alpha.,6.beta.,12a.alpha.))-
109. 2-naphthacenecarboxamide,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12a-pentahydroxy-6-methyl-1,11-dioxo-, Monohydrochloride
110. 2-naphthacenecarboxamide,7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, Monohydrochloride,(4s,4as,5as,6s,12as)-
111. 7-chloro-4-dimethylamino-1,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacene-carboxamide, Hydrochloride
| Molecular Weight | 515.3 g/mol |
|---|---|
| Molecular Formula | C22H24Cl2N2O8 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 2 |
| Exact Mass | 514.0909711 g/mol |
| Monoisotopic Mass | 514.0909711 g/mol |
| Topological Polar Surface Area | 182 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 1010 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Antiprotozoal Agents
Substances that are destructive to protozoans. (See all compounds classified as Antiprotozoal Agents.)
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?