
Synopsis
Synopsis
0
CEP/COS
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
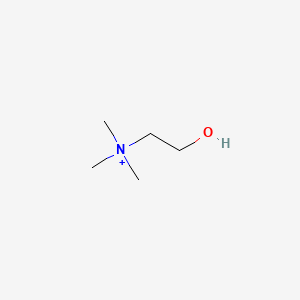

1. 2-hydroxy-n,n,n-trimethylethanaminium
2. Bitartrate, Choline
3. Bursine
4. Chloride, Choline
5. Choline Bitartrate
6. Choline Chloride
7. Choline Citrate
8. Choline Hydroxide
9. Choline O Sulfate
10. Choline O-sulfate
11. Citrate, Choline
12. Fagine
13. Hydroxide, Choline
14. O-sulfate, Choline
15. Vidine
1. Choline Ion
2. Bilineurine
3. 62-49-7
4. Choline Cation
5. 2-hydroxy-n,n,n-trimethylethanaminium
6. Cholinum
7. Ethanaminium, 2-hydroxy-n,n,n-trimethyl-
8. (2-hydroxyethyl)trimethylammonium
9. Trimethylethanolamine
10. N-trimethylethanolamine
11. Vitamin J
12. 2-hydroxyethyl(trimethyl)azanium
13. Ccris 5847
14. Ai3-24208
15. Brn 1736748
16. N,n,n-trimethylethanol-ammonium
17. Chebi:15354
18. (beta-hydroxyethyl)trimethylammonium
19. Chembl920
20. N91bdp6h0x
21. (2-hydroxyethyl)trimethylazanium
22. 2-hydroxy-n,n,n-trimethyl-ethanaminium
23. Bilineurine; Choline Cation; Choline Ion; Nanoveson C; Vitamin J
24. Cht
25. Nsc402838
26. Ncgc00015219-03
27. Einecs 200-535-1
28. Unii-n91bdp6h0x
29. 2-hydroxyethyl(trimethyl)ammonium
30. 1oba
31. 2reg
32. 3ppq
33. Choline (dcf)
34. Choline (8ci)
35. Nanoveson C
36. Spectrum_000258
37. 2ha3
38. 3r6u
39. Choline [vandf]
40. Choline [mi]
41. Spectrum2_001938
42. Spectrum4_000867
43. Spectrum5_001579
44. Lopac-c-1754
45. Choline [who-dd]
46. Bmse000285
47. Bmse000953
48. Bmse001003
49. Epitope Id:116046
50. Schembl3142
51. Lopac0_000180
52. Kbiogr_001533
53. Kbioss_000738
54. 3-04-00-00651 (beilstein Handbook Reference)
55. Divk1c_000107
56. N,n,n-trimethylethanolammonium
57. Spbio_001975
58. Gtpl4551
59. Dtxsid8043789
60. Kbio1_000107
61. Kbio2_000738
62. Kbio2_003306
63. Kbio2_005874
64. Nsc6393
65. Ninds_000107
66. (2-hydroxyethyl)trimethyl Ammonium
67. Mono-2-hydroxyethyltrimethylammonium
68. Nsc-6393
69. Zinc3079337
70. Bbl005532
71. Bdbm50026220
72. Stl137772
73. Akos005721137
74. 2-hydroxyethyl)trimethylammonium
75. Ccg-204275
76. Db00122
77. Idi1_000107
78. Ncgc00015219-01
79. Ncgc00015219-02
80. Ncgc00015219-04
81. Ncgc00015219-07
82. Ncgc00015219-10
83. Ncgc00162082-01
84. Carbachol Impurity A [ep Impurity]
85. Sbi-0050168.p003
86. 2-hydroxy-n,n,n-trimethylammonium Chloride
87. (.beta.-hydroxyethyl)trimethylammonium
88. C00114
89. D07690
90. Ab00053822_02
91. Ethanaminium, 2-hydroxy-n,n,n-trimethyl- (9ci)
92. Q193166
93. Acetylcholine Chloride Impurity A [ep Impurity]
| Molecular Weight | 104.17 g/mol |
|---|---|
| Molecular Formula | C5H14NO+ |
| XLogP3 | -0.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 104.107539070 g/mol |
| Monoisotopic Mass | 104.107539070 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 1 |
| Complexity | 46.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For nutritional supplementation, also for treating dietary shortage or imbalance
This compound is needed for good nerve conduction throughout the CNS (central nervous system) as it is a precursor to acetylcholine (ACh). Choline is also needed for gallbladder regulation, liver function and lecithin (a key lipid) formation. Choline also aids in fat and cholesterol metabolism and prevents excessive fat build up in the liver. Choline has been used to mitigate the effects of Parkinsonism and tardive dyskinesia. Choline deficiencies may result in excessive build-up of fat in the liver, high blood pressure, gastric ulcers, kidney and liver dysfunction and stunted growth.
Lipotropic Agents
Endogenous factors or drugs that increase the transport and metabolism of LIPIDS including the synthesis of LIPOPROTEINS by the LIVER and their uptake by extrahepatic tissues. (See all compounds classified as Lipotropic Agents.)
Nootropic Agents
Drugs used to specifically facilitate learning or memory, particularly to prevent the cognitive deficits associated with dementias. These drugs act by a variety of mechanisms. (See all compounds classified as Nootropic Agents.)
Choline has known human metabolites that include Trimethylazanium and acetaldehyde.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Choline is a major part of the polar head group of phosphatidylcholine. Phosphatidylcholine's role in the maintenance of cell membrane integrity is vital to all of the basic biological processes: information flow, intracellular communication and bioenergetics. Inadequate choline intake would negatively affect all these processes. Choline is also a major part of another membrane phospholipid, sphingomyelin, also important for the maintenance of cell structure and function. It is noteworthy and not surprising that choline deficiency in cell culture causes apoptosis or programmed cell death. This appears to be due to abnormalities in cell membrane phosphatidylcholine content and an increase in ceramide, a precursor, as well as a metabolite, of sphingomyelin. Ceramide accumulation, which is caused by choline deficiency, appears to activate Caspase, a type of enzyme that mediates apoptosis. Betaine or trimethylglycine is derived from choline via an oxidation reaction. Betaine is one of the factors that maintains low levels of homocysteine by resynthesizing L-methionine from homocysteine. Elevated homocysteine levels are a significant risk factor for atherosclerosis, as well as other cardiovascular and neurological disorders. Acetylcholine is one of the major neurotransmitters and requires choline for its synthesis. Adequate acetylcholine levels in the brain are believed to be protective against certain types of dementia, including Alzheimer's disease.
 PI Health Sciences: Biotech for Hire providing end-to-end discovery and development powered by chemistry, biology, and AI.
PI Health Sciences: Biotech for Hire providing end-to-end discovery and development powered by chemistry, biology, and AI.
 Siegfried – A global CDMO delivering integrated pharmaceutical development and manufacturing solutions.
Siegfried – A global CDMO delivering integrated pharmaceutical development and manufacturing solutions.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7329
Submission : 1988-02-12
Status : Active
Type : II

NDC Package Code : 17381-251
Start Marketing Date : 2010-04-08
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16987
Submission : 2003-11-14
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20764
Submission : 2007-08-08
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22208
Submission : 2008-11-19
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3475
Submission : 1978-12-22
Status : Inactive
Type : II


Registrant Name : Pharmapia Co., Ltd.
Registration Date : 2023-07-25
Registration Number : 20230313-211-J-1459(1)
Manufacturer Name : Bajaj Healthcare Limited (Unit-2)
Manufacturer Address : Block No. 588, Savli Karachia Road, At & Post - Gothada, Tal-Savli, Dist - Vadodara - 391 776, India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Siegfried – A global CDMO delivering integrated pharmaceutical development and manufacturing solutions.
Siegfried – A global CDMO delivering integrated pharmaceutical development and manufacturing solutions.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7329
Submission : 1988-02-12
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 27301
Submission : 2013-10-01
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4013
Submission : 1980-12-15
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 24027
Submission : 2010-08-05
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22208
Submission : 2008-11-19
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20764
Submission : 2007-08-08
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16987
Submission : 2003-11-14
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3475
Submission : 1978-12-22
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3972
Submission : 1980-09-12
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Phosphatidylcholine (ethanol solution)
Registration Number : 229MF10002
Registrant's Address : Nattermannallee 1, D-50829 Cologne, Germany
Initial Date of Registration : 2017-01-10
Latest Date of Registration : 2017-01-10

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Date of Issue : 2025-07-04
Valid Till : 2028-07-03
Written Confirmation Number : WC-0277
Address of the Firm : Block No. 588, Savli Karachia Road, At & Post-Gothada-391776, Tal- Savli, Dist- ...

Choline Salicylate Solution BP
Date of Issue : 2024-12-20
Valid Till : 2027-12-19
Written Confirmation Number : WC-0599
Address of the Firm : Plot No. 15B6, APSEZ, De- Notified Area, Atchutapuram, Krishnampalem (V), Rambil...

Choline Salicylate Solution BP
Date of Issue : 2022-08-29
Valid Till : 2025-07-26
Written Confirmation Number : WC-0180
Address of the Firm : D-123, Phase -III, IDA., Jeedimetla (V), Quthbullapur (M), Medchal-Malkajgiri Di...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registrant Name : HiQ Farm Co., Ltd.
Registration Date : 2023-11-15
Registration Number : 20230313-211-J-1459(3)
Manufacturer Name : Bajaj Healthcare Limited (Un...
Manufacturer Address : Block No. 588, Savli Karachia Road, At & Post - Gothada, Tal-Savli, Dist - Vadodara, ...

Registrant Name : Agerson Bio Co., Ltd.
Registration Date : 2023-03-13
Registration Number : 20230313-211-J-1459
Manufacturer Name : Bajaj Healthcare Limited (Un...
Manufacturer Address : Block No. 588, Savli Karachia Road, At & Post - Gothada, Tal-Savli, Dist - Vadodara, ...

Registrant Name : Toru Co., Ltd.
Registration Date : 2023-07-28
Registration Number : 20230313-211-J-1459(2)
Manufacturer Name : Bajaj Healthcare Limited (Un...
Manufacturer Address : Block No. 588, Savli Karachia Road, At & Post - Gothada, Tal-Savli, Dist - Vadodara -...

Registrant Name : Pharmapia Co., Ltd.
Registration Date : 2023-07-25
Registration Number : 20230313-211-J-1459(1)
Manufacturer Name : Bajaj Healthcare Limited (Un...
Manufacturer Address : Block No. 588, Savli Karachia Road, At & Post - Gothada, Tal-Savli, Dist - Vadodara -...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Siegfried – A global CDMO delivering integrated pharmaceutical development and manufacturing solutions.
Siegfried – A global CDMO delivering integrated pharmaceutical development and manufacturing solutions.
NDC Package Code : 17381-251
Start Marketing Date : 2010-04-08
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]VMF Number : 5889
Submission : 2007-04-17
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
About the Company : HRV Pharma is a global manufacturer, seller, and exporter of APIs, intermediates, pellets, food-grade chemicals, food additives, and food ingredients. The company provides sourcing...
 PI Health Sciences: Biotech for Hire providing end-to-end discovery and development powered by chemistry, biology, and AI.
PI Health Sciences: Biotech for Hire providing end-to-end discovery and development powered by chemistry, biology, and AI.
About the Company : PI Health Sciences offers end-to-end drug discovery and development, integrating medicinal chemistry, synthetic chemistry, biology, and AI-driven technologies. Its co-located teams...
About the Company : Minakem Montreal is developing and manufacturing small molecules APIs and advanced intermediates, including corticosteroids. Following efficient processes and methodologies, our em...
About the Company : Established in 1991, Pharm-Rx is an importer and distributor of active ingredients serving the pharmaceutical, nutritional supplement, and food industries. The company follows a hi...
 Siegfried – A global CDMO delivering integrated pharmaceutical development and manufacturing solutions.
Siegfried – A global CDMO delivering integrated pharmaceutical development and manufacturing solutions.
About the Company : Siegfried is a global Contract Development and Manufacturing Organization providing integrated services for pharmaceutical ingredients & finished dosage forms. With 13 production s...
About the Company : AROCHEM INDUSTRIES is a firm incepted in the year 1978 and is engaged in the business of manufacture and sell specialty organic chemicals, bulk drugs and other organic intermediate...

About the Company : AROCHEM INDUSTRIES is a firm incepted in the year 1978 and is engaged in the business of manufacture and sell specialty organic chemicals, bulk drugs and other organic intermediate...

About the Company : AROCHEM INDUSTRIES is a firm incepted in the year 1978 and is engaged in the business of manufacture and sell specialty organic chemicals, bulk drugs and other organic intermediate...

About the Company : Bajaj Healthcare Ltd. is a company established in 1993, services various Pharmaceuticals, Nutraceutical and Food industries globally with a spirit of scrupulousness. Bajaj Healt...

About the Company : At Gnosis by Lesaffre, we harness the power of microorganisms to transform compounds into usable nutritional actives, probiotics, and nutritional yeasts that benefit human wellbein...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

Reply
21 Feb 2026
Reply
20 Aug 2025
Reply
06 Jun 2025
Reply
03 Mar 2025
Reply
25 Feb 2025
Reply
18 Dec 2024
Reply
16 May 2024
Reply
18 Oct 2023
Reply
02 Sep 2023
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Reply
12 Mar 2020
Reply
04 Mar 2020
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
22
PharmaCompass offers a list of Choline API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Choline manufacturer or Choline supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Choline manufacturer or Choline supplier.
PharmaCompass also assists you with knowing the Choline API Price utilized in the formulation of products. Choline API Price is not always fixed or binding as the Choline Price is obtained through a variety of data sources. The Choline Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Choline Salicylate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Choline Salicylate, including repackagers and relabelers. The FDA regulates Choline Salicylate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Choline Salicylate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Choline Salicylate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Choline Salicylate supplier is an individual or a company that provides Choline Salicylate active pharmaceutical ingredient (API) or Choline Salicylate finished formulations upon request. The Choline Salicylate suppliers may include Choline Salicylate API manufacturers, exporters, distributors and traders.
click here to find a list of Choline Salicylate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Choline Salicylate DMF (Drug Master File) is a document detailing the whole manufacturing process of Choline Salicylate active pharmaceutical ingredient (API) in detail. Different forms of Choline Salicylate DMFs exist exist since differing nations have different regulations, such as Choline Salicylate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Choline Salicylate DMF submitted to regulatory agencies in the US is known as a USDMF. Choline Salicylate USDMF includes data on Choline Salicylate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Choline Salicylate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Choline Salicylate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Choline Salicylate Drug Master File in Japan (Choline Salicylate JDMF) empowers Choline Salicylate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Choline Salicylate JDMF during the approval evaluation for pharmaceutical products. At the time of Choline Salicylate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Choline Salicylate suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Choline Salicylate Drug Master File in Korea (Choline Salicylate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Choline Salicylate. The MFDS reviews the Choline Salicylate KDMF as part of the drug registration process and uses the information provided in the Choline Salicylate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Choline Salicylate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Choline Salicylate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Choline Salicylate suppliers with KDMF on PharmaCompass.
A Choline Salicylate written confirmation (Choline Salicylate WC) is an official document issued by a regulatory agency to a Choline Salicylate manufacturer, verifying that the manufacturing facility of a Choline Salicylate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Choline Salicylate APIs or Choline Salicylate finished pharmaceutical products to another nation, regulatory agencies frequently require a Choline Salicylate WC (written confirmation) as part of the regulatory process.
click here to find a list of Choline Salicylate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Choline Salicylate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Choline Salicylate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Choline Salicylate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Choline Salicylate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Choline Salicylate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Choline Salicylate suppliers with NDC on PharmaCompass.
Choline Salicylate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Choline Salicylate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Choline Salicylate GMP manufacturer or Choline Salicylate GMP API supplier for your needs.
A Choline Salicylate CoA (Certificate of Analysis) is a formal document that attests to Choline Salicylate's compliance with Choline Salicylate specifications and serves as a tool for batch-level quality control.
Choline Salicylate CoA mostly includes findings from lab analyses of a specific batch. For each Choline Salicylate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Choline Salicylate may be tested according to a variety of international standards, such as European Pharmacopoeia (Choline Salicylate EP), Choline Salicylate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Choline Salicylate USP).

