
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
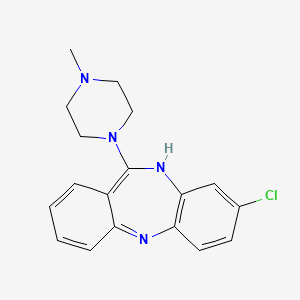

| Molecular Weight | 326.8 g/mol |
|---|---|
| Molecular Formula | C18H19ClN4 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 326.1298243 g/mol |
| Monoisotopic Mass | 326.1298243 g/mol |
| Topological Polar Surface Area | 30.9 A^2 |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 584 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Clozapine |
| PubMed Health | Clozapine (By mouth) |
| Drug Classes | Antipsychotic |
| Drug Label | VERSACLOZ, an atypical antipsychotic drug, is a tricyclic dibenzodiazepine derivative, 8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine.The structural formula is: VERSACLOZ is available as a free-flowing yellow suspension. Each mL c... |
| Active Ingredient | Clozapine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 100mg; 25mg; 50mg; 12.5mg |
| Market Status | Prescription |
| Company | Ivax Sub Teva Pharms; Sun Pharm Inds; Mylan |
| 2 of 6 | |
|---|---|
| Drug Name | Clozaril |
| PubMed Health | Clozapine (By mouth) |
| Drug Classes | Antipsychotic |
| Drug Label | CLOZARIL (clozapine), an atypical antipsychotic drug, is a tricyclic dibenzodiazepine derivative, 8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo [b,e] [1,4] diazepine.The structural formula isCLOZARIL is available in pale yellow tablets of 25mg... |
| Active Ingredient | Clozapine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg |
| Market Status | Prescription |
| Company | Novartis |
| 3 of 6 | |
|---|---|
| Drug Name | Fazaclo odt |
| Active Ingredient | Clozapine |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 200mg; 100mg; 25mg; 150mg; 12.5mg |
| Market Status | Prescription |
| Company | Jazz Pharms Iii |
| 4 of 6 | |
|---|---|
| Drug Name | Clozapine |
| PubMed Health | Clozapine (By mouth) |
| Drug Classes | Antipsychotic |
| Drug Label | VERSACLOZ, an atypical antipsychotic drug, is a tricyclic dibenzodiazepine derivative, 8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine.The structural formula is: VERSACLOZ is available as a free-flowing yellow suspension. Each mL c... |
| Active Ingredient | Clozapine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 100mg; 25mg; 50mg; 12.5mg |
| Market Status | Prescription |
| Company | Ivax Sub Teva Pharms; Sun Pharm Inds; Mylan |
| 5 of 6 | |
|---|---|
| Drug Name | Clozaril |
| PubMed Health | Clozapine (By mouth) |
| Drug Classes | Antipsychotic |
| Drug Label | CLOZARIL (clozapine), an atypical antipsychotic drug, is a tricyclic dibenzodiazepine derivative, 8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo [b,e] [1,4] diazepine.The structural formula isCLOZARIL is available in pale yellow tablets of 25mg... |
| Active Ingredient | Clozapine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg |
| Market Status | Prescription |
| Company | Novartis |
| 6 of 6 | |
|---|---|
| Drug Name | Fazaclo odt |
| Active Ingredient | Clozapine |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 200mg; 100mg; 25mg; 150mg; 12.5mg |
| Market Status | Prescription |
| Company | Jazz Pharms Iii |

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code :
Brand Name : CLOZAPINE
Dosage Form : TABLET;ORAL
Dosage Strength : 25MG
Approval Date : 2015-09-17
Application Number : 203807
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code :
Brand Name : CLOZAPINE
Dosage Form : TABLET;ORAL
Dosage Strength : 100MG
Approval Date : 2015-09-17
Application Number : 203807
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code :
Brand Name : CLOZAPINE
Dosage Form : TABLET;ORAL
Dosage Strength : 50MG
Approval Date : 2017-08-22
Application Number : 203807
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code :
Brand Name : CLOZAPINE
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG
Approval Date : 2017-08-22
Application Number : 203807
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : CLOZAPINE
Dosage Form : TABLET;ORAL
Dosage Strength : 25MG
Approval Date : 2015-11-25
Application Number : 202873
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : CLOZAPINE
Dosage Form : TABLET, ORALLY DISINTEGRATING;ORAL
Dosage Strength : 100MG
Approval Date : 2015-11-25
Application Number : 90308
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : CLOZAPINE
Dosage Form : TABLET;ORAL
Dosage Strength : 100MG
Approval Date : 1997-11-26
Application Number : 74949
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : FAZACLO ODT
Dosage Form : TABLET, ORALLY DISINTEGRATING;ORAL
Dosage Strength : 100MG **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Approval Date : 2004-02-10
Application Number : 21590
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : CLOZAPINE
Dosage Form : TABLET;ORAL
Dosage Strength : 25MG
Approval Date : 2002-11-15
Application Number : 75713
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : CLOZAPINE
Dosage Form : TABLET, ORALLY DISINTEGRATING;ORAL
Dosage Strength : 200MG
Approval Date : 2023-08-07
Application Number : 201824
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?