
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
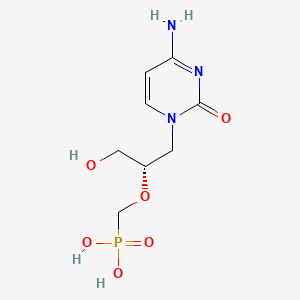

1. 1-((3-hydroxy-2-phosphonylmethoxy)propyl)cytosine
2. 1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine
3. Cidofovir Anhydrous
4. Cidofovir Sodium
5. Cidofovir, (+-)-isomer
6. Cidofovir, (r)-isomer
7. Cidofovir, Sodium Salt
8. Gs 504
9. Gs-504
10. Gs504
11. Hpmpc
12. Vistide
1. 113852-37-2
2. Vistide
3. Hpmpc
4. Cidofovir Anhydrous
5. (s)-hpmpc
6. Gs-504
7. Anhydrous Cidofovir
8. Cidofovir [inn]
9. Cidofovir (vistide)
10. Cidofovir Hydrate
11. Gs 0504
12. (s)-1-(3-hydroxy-2-phosphonomethoxypropyl)cytosine
13. Cdv
14. [1-(4-amino-2-oxo-pyrimidin-1-yl)-3-hydroxy-propan-2-yl]oxymethylphosphonic Acid
15. Gs-0504
16. (s)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine
17. (s)-(((1-(4-amino-2-oxopyrimidin-1(2h)-yl)-3-hydroxypropan-2-yl)oxy)methyl)phosphonic Acid
18. Chebi:3696
19. Hpmpc Dihydrate
20. ({[(2s)-1-(4-amino-2-oxopyrimidin-1(2h)-yl)-3-hydroxypropan-2-yl]oxy}methyl)phosphonic Acid
21. (s)-1-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine
22. 768m1v522c
23. Nsc-742135
24. Ncgc00184994-01
25. Phosphonic Acid, ((2-(4-amino-2-oxo-1(2h)-pyrimidinyl)-1-(hydroxymethyl)ethoxy)methyl)-, (s)-
26. 1-(s)-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine
27. 1-[(s)-3-hydroxy-2-(phosphonomethoxy)propyl]cytosine
28. Cidofovir (anhydrous)
29. Cidofovir Hydrate (1:2)
30. Cidofovirum
31. Forvade
32. (s)-(3-(4-amino-2-oxopyrimidin-1(2h)-yl)-1-hydroxypropan-2-yloxy)methylphosphonic Acid
33. [(s)-2-(4-amino-2-oxo-2h-pyrimidin-1-yl)-1-hydroxymethyl-ethoxymethyl]-phosphonic Acid
34. ({[(2s)-1-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3-hydroxypropan-2-yl]oxy}methyl)phosphonic Acid
35. Hsdb 7115
36. Gs504
37. 1-((s)-3-hydroxy-2-(phosphonomethoxy)propyl)cytosine
38. Cidofovir Gel
39. Unii-768m1v522c
40. Cidofovir,vistide
41. [[(s)-2-(4-amino-2-oxo-1(2h)-pyrimidinyl)-1-(hydroxymethyl)ethoxy]methyl]phosphonic Acid
42. Cidofovir(vistide)
43. (((s)-2-(4-amino-2-oxo-1(2h)-pyrimidinyl)-1-(hydroxymethyl)ethoxy)methyl)phosphonic Acid
44. Forvade (tm)
45. Cidofovir [mi]
46. Cidofovir [hsdb]
47. Chembl152
48. Cidofovir [who-dd]
49. Cidofovir Anhydrous- Bio-x
50. Schembl3948
51. Dsstox_cid_23734
52. Dsstox_rid_80069
53. Dsstox_gsid_43734
54. (2s)-3-hydroxy-2-phosphonylmethoxypropyl-cytosine
55. Mls003915629
56. (s)-1-[3-hydroxy-2-(phosphonylmethoxy)-propyl]cytosine
57. Dtxsid3043734
58. Bdbm31915
59. 1-[(s)-3-hydroxy-2-(phosphonomethoxy)propyl]-cytosine Dihydrate
60. Bcp03734
61. Ex-a4209
62. Zinc1530600
63. Tox21_112994
64. Mfcd00866936
65. Nsc742135
66. S1516
67. Akos005145721
68. Akos015854828
69. Ac-1666
70. Bcp9000528
71. Ccg-267235
72. Cs-1669
73. Db00369
74. Gs-6438
75. (s)-2-(4-amino-2-oxo-1(2h)-pyrimidinyl-1-(hydroxymethyl)ethoxy)methyl Phosphonic Acid
76. [(1s)-1-[(4-amino-2-oxo-pyrimidin-1-yl)methyl]-2-hydroxy-ethoxy]methylphosphonic Acid
77. Ncgc00184994-02
78. Ncgc00184994-03
79. Bc164304
80. Hy-17438
81. Smr002544687
82. Bcp0726000147
83. Cas-113852-37-2
84. Ab01566823_01
85. 394c661
86. Q423445
87. Sr-01000931969
88. J-502695
89. Sr-01000931969-2
90. (s)-(((1-(4-amino-2-oxopyrimidin-1(2h)-yl)-3-hydroxypropan-2-yl)oxy)methyl)phosphonicacid
91. ({[(s)-1-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3-hydroxypropan-2-yl]oxy}methyl)phosphonic Acid
92. L8p
93. Phosphonic Acid, [[(1s)-2-(4-amino-2-oxo-1(2h)-pyrimidinyl)-1-(hydroxymethyl)ethoxy]methyl]-
94. Phosphonic Acid,[[(1s)-2-(4-amino-2-oxo-1(2h)-pyrimidinyl)-1-(hydroxymethyl)ethoxy]methyl]-
| Molecular Weight | 279.19 g/mol |
|---|---|
| Molecular Formula | C8H14N3O6P |
| XLogP3 | -3.6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 279.06202217 g/mol |
| Monoisotopic Mass | 279.06202217 g/mol |
| Topological Polar Surface Area | 146 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 417 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Cidofovir |
| PubMed Health | Cidofovir (Injection) |
| Drug Classes | Antiviral |
| Drug Label | The chemical name of cidofovir is 1-[(S)-3-hydroxy-2-(phosphonomethoxy)propyl]cytosine dihydrate (HPMPC), with the molecular formula of C8H14N3O6P2H2O and a molecular weight of 315.22 (279.19 for anhydrous). The chemical structure is:Cidofovir is... |
| Active Ingredient | Cidofovir |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 75mg base/ml |
| Market Status | Prescription |
| Company | Emcure Pharms; Mylan Institutional |
| 2 of 4 | |
|---|---|
| Drug Name | Vistide |
| PubMed Health | Cidofovir (Injection) |
| Drug Classes | Antiviral |
| Drug Label | VISTIDE is the brand name for cidofovir injection. The chemical name of cidofovir is 1-[(S)-3-hydroxy-2-(phosphonomethoxy)propyl]cytosine dihydrate (HPMPC), with the molecular formula of C8H14N3O6P2H2O and a molecular weight of 315.22 (279.19 fo... |
| Active Ingredient | Cidofovir |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 75mg base/ml |
| Market Status | Prescription |
| Company | Gilead Sciences |
| 3 of 4 | |
|---|---|
| Drug Name | Cidofovir |
| PubMed Health | Cidofovir (Injection) |
| Drug Classes | Antiviral |
| Drug Label | The chemical name of cidofovir is 1-[(S)-3-hydroxy-2-(phosphonomethoxy)propyl]cytosine dihydrate (HPMPC), with the molecular formula of C8H14N3O6P2H2O and a molecular weight of 315.22 (279.19 for anhydrous). The chemical structure is:Cidofovir is... |
| Active Ingredient | Cidofovir |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 75mg base/ml |
| Market Status | Prescription |
| Company | Emcure Pharms; Mylan Institutional |
| 4 of 4 | |
|---|---|
| Drug Name | Vistide |
| PubMed Health | Cidofovir (Injection) |
| Drug Classes | Antiviral |
| Drug Label | VISTIDE is the brand name for cidofovir injection. The chemical name of cidofovir is 1-[(S)-3-hydroxy-2-(phosphonomethoxy)propyl]cytosine dihydrate (HPMPC), with the molecular formula of C8H14N3O6P2H2O and a molecular weight of 315.22 (279.19 fo... |
| Active Ingredient | Cidofovir |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 75mg base/ml |
| Market Status | Prescription |
| Company | Gilead Sciences |
Cidofovir is used for the treatment of cytomegalovirus (CMV) retinitis in patients with acquired immunodeficiency syndrome (AIDS).
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 538
Cidofovir has been used for the management of acyclovir-resistant herpes simplex virus (HSV-1 and HSV-2) infections in immunocompromised patients.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 539
The role, if any, of cidofovir in the treatment of smallpox remains to be determined. Cidofovir is active in vitro against poxviruses, including variola virus (the causative agent of smallpox) and has in vivo activity in mice against cowpox and vaccinia virus. Although limited in vitro and in vivo data suggest that cidofovir might prove useful in preventing smallpox infection if administered within 1-2 days after exposure, there currently is no evidence that the antiviral would be more effective than vaccination in this early period.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 539
Topical cidofovir gel eliminates virus shedding and lesions in some HIV-infected patients with acyclovir-resistant mucocutaneous HSV infections and has been use in treating anogenital warts and molluscum contagiosum in immunocompromised patients and cervical intraepithelial neoplasia in women. Intralesional cidofovir induces remission in adults or chlidren with respiratory papillomatosis.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1322
For more Therapeutic Uses (Complete) data for CIDOFOVIR (15 total), please visit the HSDB record page.
Placement of a ganciclovir ocular implant in patients receiving IV cidofovir has resulted in profound hypotomy in some patients, and some clinicians suggest that IV cidofovir not be administered within one month before or after placement of a ganciclovir ocular implant.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 539
.... Because evidence from clinical trials indicates that previous exposure to foscarnet may increase the risk of cidofovir-related nephrotoxicity, patients treated previously with foscarnet should receive cidofovir only when the potential benefits exceed the possible risks.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 539
Patients should be advised that cidofovir therapy is not curative, and that progression of their retinitis is possible during or following therapy with the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 538
Safety and efficacy of cidofovir in geriatric patients older than 60 years of age have not been established. Because geriatric patients frequently have reduced glomerular filtration, particular attention should be paid to monitoring renal function prior to and during cidofovir therapy in this age group, and doses of the drug should be modified in response to changes in renal function that occur during therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 539
For more Drug Warnings (Complete) data for CIDOFOVIR (20 total), please visit the HSDB record page.
For the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS)
FDA Label
Vistide is indicated for the treatment of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome (AIDS) and without renal dysfunction. Vistide should be used only when other agents are considered unsuitable.
Cidofovir is a new anti-viral drug. It is classified as a nucleotide analogue and is active against herpes cytomegalovirus (CMV) retinitis infection. Most adults are infected with CMV. Cidofovir suppresses cytomegalovirus (CMV) replication by selective inhibition of viral DNA synthesis.
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
J05AB12
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AB - Nucleosides and nucleotides excl. reverse transcriptase inhibitors
J05AB12 - Cidofovir
Absorption
100%
Volume of Distribution
537 126 mL/kg [VISTIDE ADMINISTERED WITHOUT PROBENECID]
410 102 mL/kg [VISTIDE ADMINISTERED WITH PROBENECID]
Clearance
179 +/- 23.1 mL/min/1.73 m2 [WITHOUT PROBENECID]
148 +/- 38.8 mL/min/1.73 m2 [WITH PROBENECID]
Volume of distribution is 537 ml/kg without concurrent probenecid administration and 410 ml/kg with concurrent probenecid administration.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 879
Concentrations of cidofovir were undetectable 15 minutes after the end of a 1 hour infusion in one patient who had a corresponding serum concentration of 8.7 ug/mL.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 879
Renal (without concurrent probenecid administration): Approximately 80 to 100% of an administered cidofovir dose was recovered unchanged in the urine within 24 hours.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 879
Cidofovir is dianionic at physiological pH and have low oral bioavailability in animals and humans. After intravenous administration to HIV-infected patients, the pharmacokinetics of /cidofovir is/ independent of dose and are consistent with preclinical data. Systemic exposure is proportional to the intravenous dose and /the drug is cleared/ by the kidney and excreted extensively as unchanged drug in the urine. Intracellular activation of a small fraction (< 10%) of the dose by cellular kinases leads to prolonged antiviral effects that are not easily predicted from conventional pharmacokinetic studies. The observed rate of elimination of cidofovir ... from the serum may not reflect the true duration of action of these drugs, since the antiviral effect is dependent on concentrations of the active phosphorylated metabolites that are present within cells. For /cidofovir/, > 90% of an iv dose is recovered unchanged in the urine over 24 hours.
PMID:10092959 Cundy KC; Clin Pharmacokinet 36 (2): 127-43 (1999)
This study was undertaken to evaluate the intravitreal and plasma concentrations of cidofovir (HPMPC) after intravitreal and intravenous administration in AIDS patients with cytomegalovirus retinitis. Cohort series; undiluted vitreous and blood were collected from 9 patients at the time of pars plana vitrectomy. Vitreous samples from 9 eyes of 9 patients and plasma samples from 4 patients were assayed with high-performance liquid chromatography to determine cidofovir levels. The only eye that had a detectable vitreous concentration (673.7 ng/ml) was injected with 20 microg 24 hours prior to the surgery. The remaining samples including plasma were below the detection point of the assay (100 ng/ml) and were injected between 5 and 40 days prior to sampling. The intravitreal concentration of cidofovir in humans is consistent with pharmacokinetics data in laboratory animals, and suggests that the long duration of antiviral effect (1-3 months) in clinical trials is due to a prolonged intracellular half-life in retinal tissue.
PMID:9572540 Taskintuna I et al; J Ocul Pharmacol Ther 14 (2): 147-51 (1998)
Cidofovir is converted via cellular enzymes to the pharmacologically active diphosphate metabolite ... .
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 540
This study was designed to evaluate the intraocular distribution and metabolism of the antiviral nucleotide analogs cidofovir and cyclic 1-[(S)-3-hydroxy-2-(phosphonomethoxy) propyl]cytosine (HPMPC) in New Zealand white rabbits following intravitreal administration. ...Male rabbits received either 14C-cidofovir or 14C-cyclic HPMPC by intravitreal injection into both eyes (50 micrograms/eye, 11 microCi/eye). Two animals/group were sacrificed at 24, 48, 72 or 240 hr post-dose. Ocular tissues, kidney and liver were oxidized to determine total radioactivity and metabolites were determined by HPLC. ...At 24 hr post-dose, total radioactivity was 9.96 and 5.18 micrograms-equiv/g for cidofovir and cyclic HPMPC, respectively, in vitreous and 20.9 and 3.54 micrograms-equiv/g, respectively, in retina. Although the initial vitreal clearance was 2-fold faster for the cyclic analog, the estimated terminal elimination half-lives in vitreous (42 hr) and in retina (66-77 hr) were similar for both drugs. By 240 hr post-dose, radioactivity in all ocular tissues was approx ten-fold higher for cidofovir. Radioactivity in vitreous at 240 hr after intravitreal dosing with either drug contained cidofovir, cyclic HPMPC and cidofovir-phosphocholine. ...The long retinal half-life observed presumably reflects formation of phosphorylated cidofovir within retinal cells. Cidofovir achieved a ten-fold higher level of phosphorylated drug in retina than cyclic HPMPC. Therefore, intravitreal cidofovir may be expected to suppress progression of retinitis for a longer period than an equivalent intravitreal dose of cyclic HPMPC. The intravitreal half-life of cidofovir was 20-fold longer than that of ganciclovir in the same animal model.
PMID:8670758 Cundy KC et al; Curr Eye Res 15 (5): 569-76 (1996)
Pyrimidine nucleoside monophosphate kinase converts cidofovir to cidofovir monophosphate, which is further converted to the diphosphate and cidofovir phosphate-choline via other cellular enzymes.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 540
2.4 to 3.2 hours
...Male rabbits received either 14C-cidofovir ...by intravitreal injection into both eyes (50 micrograms/eye...). ...The estimated terminal elimination half-lives /were/ in vitreous (42 hr) and in retina (66-77 hr)... .
PMID:8670758 Cundy KC et al; Curr Eye Res 15 (5): 569-76 (1996)
...Levels of cidofovir in serum following iv infusion were dose proportional over the dose range of 1.0-10.0 mg/kg bw and declined biexponentially with an overall mean +/- standard deviation terminal half-life of 2.6 +/- 1.2 hr (n=25).
PMID:7574510 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC162721 Cundy KC et al; Antimicrob Agents Chemother 39 (6): 1247-52 (1995)
Cidofovir acts through the selective inhibition of viral DNA polymerase.Biochemical data support selective inhibition of CMV DNA polymerase by cidofovir diphosphate, the active intracellular metabolite of cidofovir. Cidofovir diphosphate inhibits herpesvirus polymerases at concentrations that are 8- to 600-fold lower than those needed to inhibit human cellular DNA polymerase alpha, beta, and gamma(1,2,3). Incorporation of cidofovir into the growing viral DNA chain results in reductions in the rate of viral DNA synthesis.
Cidofovir diphosphate exerts its antiviral effect by interfering with DNA synthesis and inhibiting viral replication. The inhibitory activity of cidofovir diphosphate is highly selective because of its greater affinity for viral DNA polymerases than for human DNA polymerases. /Cidofovir diphosphate/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 540
Pyrimidine nucleoside monophosphate kinase converts cidofovir to cidofovir monophosphate, which is further converted to the diphosphate and cidofovir phosphate-choline via other cellular enzymes. Cidofovir diphosphate stop replication of viral DNA by competitive inhibition of viral DNA polymerase, incorporation and termination of the growing viral DNA chain, and inactivation of the viral DNA polymerase. /Cidofovir diphosphate/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 540
Cidofovir /is an/ ... acyclic phosphonate analog of deoxynucleoside monophosphate. /This cmpd/ undergoes intracellular activation to form diphosphates that are potent inhibitors of viral DNA polymerases. Cidofovir has broad spectrum antiviral activity against herpesviruses, papillomaviruses and poxviruses.
PMID:10092959 Cundy KC; Clin Pharmacokinet 36 (2): 127-43 (1999)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
64
PharmaCompass offers a list of Cidofovir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Cidofovir manufacturer or Cidofovir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Cidofovir manufacturer or Cidofovir supplier.
PharmaCompass also assists you with knowing the Cidofovir API Price utilized in the formulation of products. Cidofovir API Price is not always fixed or binding as the Cidofovir Price is obtained through a variety of data sources. The Cidofovir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A cidofovir anhydrous manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of cidofovir anhydrous, including repackagers and relabelers. The FDA regulates cidofovir anhydrous manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. cidofovir anhydrous API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of cidofovir anhydrous manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A cidofovir anhydrous supplier is an individual or a company that provides cidofovir anhydrous active pharmaceutical ingredient (API) or cidofovir anhydrous finished formulations upon request. The cidofovir anhydrous suppliers may include cidofovir anhydrous API manufacturers, exporters, distributors and traders.
click here to find a list of cidofovir anhydrous suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A cidofovir anhydrous DMF (Drug Master File) is a document detailing the whole manufacturing process of cidofovir anhydrous active pharmaceutical ingredient (API) in detail. Different forms of cidofovir anhydrous DMFs exist exist since differing nations have different regulations, such as cidofovir anhydrous USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A cidofovir anhydrous DMF submitted to regulatory agencies in the US is known as a USDMF. cidofovir anhydrous USDMF includes data on cidofovir anhydrous's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The cidofovir anhydrous USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of cidofovir anhydrous suppliers with USDMF on PharmaCompass.
A cidofovir anhydrous written confirmation (cidofovir anhydrous WC) is an official document issued by a regulatory agency to a cidofovir anhydrous manufacturer, verifying that the manufacturing facility of a cidofovir anhydrous active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting cidofovir anhydrous APIs or cidofovir anhydrous finished pharmaceutical products to another nation, regulatory agencies frequently require a cidofovir anhydrous WC (written confirmation) as part of the regulatory process.
click here to find a list of cidofovir anhydrous suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing cidofovir anhydrous as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for cidofovir anhydrous API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture cidofovir anhydrous as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain cidofovir anhydrous and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a cidofovir anhydrous NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of cidofovir anhydrous suppliers with NDC on PharmaCompass.
cidofovir anhydrous Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of cidofovir anhydrous GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right cidofovir anhydrous GMP manufacturer or cidofovir anhydrous GMP API supplier for your needs.
A cidofovir anhydrous CoA (Certificate of Analysis) is a formal document that attests to cidofovir anhydrous's compliance with cidofovir anhydrous specifications and serves as a tool for batch-level quality control.
cidofovir anhydrous CoA mostly includes findings from lab analyses of a specific batch. For each cidofovir anhydrous CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
cidofovir anhydrous may be tested according to a variety of international standards, such as European Pharmacopoeia (cidofovir anhydrous EP), cidofovir anhydrous JP (Japanese Pharmacopeia) and the US Pharmacopoeia (cidofovir anhydrous USP).
