
Synopsis
Synopsis
0
CEP/COS
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
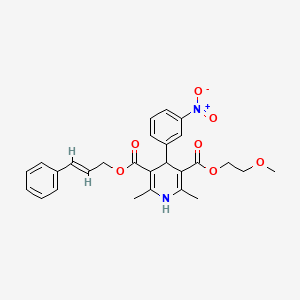

1. 2-methoxyethyl-3-phenyl-2-propen-1-yl-1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylate
2. Cilnidipine, (+)-isomer
3. Cilnidipine, (-)-isomer
4. Frc 8653
5. Frc-8653
1. 132203-70-4
2. Cinalong
3. Atelec
4. Siscard
5. Frc-8653
6. Frc 8653
7. 3-cinnamyl 5-(2-methoxyethyl) 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
8. Cilnidipine [inn]
9. Chebi:31399
10. Cilnidipine, (+)-
11. Cilnidipine, (-)-
12. (+-)-(e)-cinnamyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
13. 97t5az1jip
14. 4lnu2su262
15. S85436zg85
16. 2-methoxyethyl (2e)-3-phenylprop-2-en-1-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
17. Ncgc00162150-01
18. Cinaldipine
19. (+)-frc-8653
20. (-)-frc-8653
21. Dsstox_cid_26309
22. Dsstox_rid_81530
23. Dsstox_gsid_46309
24. (e)-cinnamyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
25. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-methoxyethyl 3-phenyl-2-propenyl Ester, (e)-(+)-
26. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-methoxyethyl 3-phenyl-2-propenyl Ester, (e)-(-)-
27. Atelec (tn)
28. Cas-132203-70-4
29. Sr-05000001454
30. 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic Acid 2-methoxyethyl (2e)-3-phenyl-2-propenyl Ester
31. Unii-97t5az1jip
32. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 3-(2-methoxyethyl) 5-((2e)-3-phenyl-2-propen-1-yl) Ester
33. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 3-(2-methoxyethyl) 5-[(2e)-3-phenyl-2-propen-1-yl] Ester
34. Cilnidipine- Bio-x
35. Mfcd00865853
36. Cilnidipine [mi]
37. Cilnidipine [jan]
38. Cilnidipine (jp17/inn)
39. Cilnidipine [mart.]
40. Unii-4lnu2su262
41. Schembl25550
42. Cilnidipine [who-dd]
43. 3-o-(2-methoxyethyl) 5-o-[(e)-3-phenylprop-2-enyl] 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
44. Chembl452076
45. Gtpl7767
46. Dtxsid0046309
47. Unii-s85436zg85
48. Chebi:91506
49. Hms2089j07
50. Hms3261e06
51. Hms3413l13
52. Hms3677l13
53. Hms3715n17
54. Hms3884k09
55. Bcp22689
56. Tox21_112001
57. Tox21_500422
58. Ac-270
59. Bdbm50101813
60. S1293
61. Stk623341
62. Akos005558085
63. Tox21_112001_1
64. Ccg-221188
65. Ccg-221726
66. Cilnidipine, >=98% (hplc), Powder
67. Cs-1133
68. Db09232
69. Ks-1294
70. Lp00422
71. Sdccgsbi-0633712.p001
72. Ncgc00162150-02
73. Ncgc00162150-03
74. Ncgc00162150-04
75. Ncgc00162150-16
76. Ncgc00261107-01
77. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-methoxyethyl 3-phenyl-2-propenyl Ester, (e)-(+-)-
78. Bc164309
79. Hy-17404
80. Ls-15175
81. Sw219784-1
82. D01173
83. T70209
84. Ab01274755-01
85. Ab01274755-02
86. Ab01274755_03
87. Frc-8653; Frc 8653; Frc8653
88. 203c704
89. Q731525
90. J-006141
91. Sr-05000001454-1
92. Sr-05000001454-2
93. Brd-a07875874-001-01-6
94. F2173-0669
95. (+/-)-(e)-cinnamyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
96. 102106-21-8
97. 118934-76-2
98. 118934-77-3
99. 132295-21-7
100. 132338-87-5
101. 2-methoxyethyl (2e)-3-phenyl-2-propenyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate
102. O3-(2-methoxyethyl) O5-(3-phenylprop-2-enyl) 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
| Molecular Weight | 492.5 g/mol |
|---|---|
| Molecular Formula | C27H28N2O7 |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 11 |
| Exact Mass | 492.18965124 g/mol |
| Monoisotopic Mass | 492.18965124 g/mol |
| Topological Polar Surface Area | 120 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 896 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cilnidipine is indicated for the management of hypertension for end-organ protection. It is reported to be useful in elderly patients and in those with diabetes and albuminuria. Cilnidipine has been increasingly used in patients with chronic kidney disease Hypertension is the term used to describe the presence of high blood pressure. The blood pressure is generated by the force of the blood pumped from the heart against the blood vessels. Thus hypertension is caused when there is too much pressure on the blood vessels and this effect can damage the blood vessel.
Administration of cilnidipine has been shown to present an antisympathetic profile in vitro and in vivo. It decreases blood pressure safely and effectively without excessive blood pressure reduction or tachycardia.
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
C - Cardiovascular system
C08 - Calcium channel blockers
C08C - Selective calcium channel blockers with mainly vascular effects
C08CA - Dihydropyridine derivatives
C08CA14 - Cilnidipine
Absorption
Cilnidipine presents a very rapid absorption with a maximum peaked concentration after 2 hours. Its distribution tends to be higher in the liver as well as in kidneys, plasma and other tissues. Cilnidipine does not present a high accumulation in the tissue after repeated oral administration. Cilnidipine is reported to present very low bioavailability determined to be approximately 13%. This low bioavailability is suggested to be due to its low aqueous solubility and high permeability. Hence, efforts have been made in order to find an innovative formulation that can significantly improve the bioavailability of this drug. One of these formulations corresponds to the generation of polymeric nanoparticles which enhance the bioavailability by 2.5-3-fold.
Route of Elimination
Cilnidipine gets eliminated through the urine in a proportion of 20% of the administered dose and 80% is eliminated by the feces.
Volume of Distribution
Drugs on the group of dihydropyridines such as cilnidipine tend to have a large volume of distribution.
Cilnidipine is metabolized by both liver and kidney. It is rapidly metabolized by liver microsomes by a dehydrogenation process. The major enzymatic isoform involved in cilnidipine dehydrogenation of the dihydropyridine ring is CYP3A.
The half-life of the hypotensive effect for cilnidipine is of about 20.4 min.
Cilnidipine acts on the L-type calcium channels of blood vessels by blocking the incoming calcium and suppressing the contraction of blood vessels, thereby reducing blood pressure. Cilnidipine also works on the N-type calcium channel located at the end of the sympathetic nerve, inhibiting the emission of norepinephrine and suppressing the increase in stress blood pressure.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : Telmaneta Cd
Dosage Form : Tablet
Dosage Strength : 40MG; 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 5MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 40MG; 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 5MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 20MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : Telmivaz LN
Dosage Form : Tablet
Dosage Strength : 40MG; 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Korea
Brand Name : CINAPIN
Dosage Form : TABLET
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Korea

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : Dipibloc
Dosage Form : Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : Telmaneta Cd
Dosage Form : Tablet
Dosage Strength : 40MG; 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 40MG; 10MG
Brand Name : Telmaneta Cd
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 5MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 5MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 10MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 40MG; 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 40MG; 10MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 10MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 20MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 20MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : Telmivaz LN
Dosage Form : Tablet
Dosage Strength : 40MG; 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 40MG; 10MG
Brand Name : Telmivaz LN
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Telmisartan; Cilnidipine; Metoprolol
Brand Name : Telmivaz 3D25
Dosage Form : Tablet
Dosage Strength : 40MG; 10MG; 25MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Telmisartan; Cilnidipine; Metoprolol
Dosage : Tablet
Dosage Strength : 40MG; 10MG; 25MG
Brand Name : Telmivaz 3D25
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Korea
Brand Name : CINAPIN
Dosage Form : TABLET
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Korea

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : TABLET
Dosage Strength : 10MG
Brand Name : CINAPIN
Approval Date :
Application Number :
Registration Country : South Korea

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : Dipibloc
Dosage Form : Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 10MG
Brand Name : Dipibloc
Approval Date :
Application Number :
Registration Country : India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
13 Jun 2022
Reply
06 Jun 2016
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
ABOUT THIS PAGE
77
PharmaCompass offers a list of Cilnidipine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Cilnidipine manufacturer or Cilnidipine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Cilnidipine manufacturer or Cilnidipine supplier.
PharmaCompass also assists you with knowing the Cilnidipine API Price utilized in the formulation of products. Cilnidipine API Price is not always fixed or binding as the Cilnidipine Price is obtained through a variety of data sources. The Cilnidipine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Cilnidipine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Cilnidipine, including repackagers and relabelers. The FDA regulates Cilnidipine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Cilnidipine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Cilnidipine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Cilnidipine supplier is an individual or a company that provides Cilnidipine active pharmaceutical ingredient (API) or Cilnidipine finished formulations upon request. The Cilnidipine suppliers may include Cilnidipine API manufacturers, exporters, distributors and traders.
click here to find a list of Cilnidipine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Cilnidipine DMF (Drug Master File) is a document detailing the whole manufacturing process of Cilnidipine active pharmaceutical ingredient (API) in detail. Different forms of Cilnidipine DMFs exist exist since differing nations have different regulations, such as Cilnidipine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Cilnidipine DMF submitted to regulatory agencies in the US is known as a USDMF. Cilnidipine USDMF includes data on Cilnidipine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Cilnidipine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Cilnidipine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Cilnidipine Drug Master File in Japan (Cilnidipine JDMF) empowers Cilnidipine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Cilnidipine JDMF during the approval evaluation for pharmaceutical products. At the time of Cilnidipine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Cilnidipine suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Cilnidipine Drug Master File in Korea (Cilnidipine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Cilnidipine. The MFDS reviews the Cilnidipine KDMF as part of the drug registration process and uses the information provided in the Cilnidipine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Cilnidipine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Cilnidipine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Cilnidipine suppliers with KDMF on PharmaCompass.
A Cilnidipine written confirmation (Cilnidipine WC) is an official document issued by a regulatory agency to a Cilnidipine manufacturer, verifying that the manufacturing facility of a Cilnidipine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Cilnidipine APIs or Cilnidipine finished pharmaceutical products to another nation, regulatory agencies frequently require a Cilnidipine WC (written confirmation) as part of the regulatory process.
click here to find a list of Cilnidipine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Cilnidipine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Cilnidipine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Cilnidipine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Cilnidipine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Cilnidipine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Cilnidipine suppliers with NDC on PharmaCompass.
Cilnidipine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Cilnidipine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Cilnidipine GMP manufacturer or Cilnidipine GMP API supplier for your needs.
A Cilnidipine CoA (Certificate of Analysis) is a formal document that attests to Cilnidipine's compliance with Cilnidipine specifications and serves as a tool for batch-level quality control.
Cilnidipine CoA mostly includes findings from lab analyses of a specific batch. For each Cilnidipine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Cilnidipine may be tested according to a variety of international standards, such as European Pharmacopoeia (Cilnidipine EP), Cilnidipine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Cilnidipine USP).

