
Synopsis
Synopsis
0
CEP/COS
0
VMF
0
FDF
0
Canada

0
Australia

0
South Africa

0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
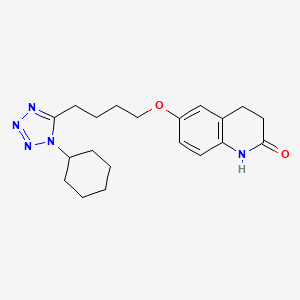

1. 6-(4-(1-cyclohexyl-1h-tetrazol-5-yl)butoxy)-3,4-dihydro-2(1h)-quinolinone
2. Opc 13013
3. Opc-13013
4. Pletal
1. 73963-72-1
2. Pletal
3. Cilostazole
4. Pletaal
5. Opc-13013
6. Cilostazolum
7. Opc-21
8. Cilostazolum [inn-latin]
9. Opc 13013
10. Opc 21
11. 6-[4-(1-cyclohexyltetrazol-5-yl)butoxy]-3,4-dihydro-1h-quinolin-2-one
12. 6-(4-(1-cyclohexyl-1h-tetrazol-5-yl)butoxy)-3,4-dihydro-2(1h)-quinolinone
13. 6-(4-(1-cyclohexyl-1h-tetrazol-5-yl)butoxy)-3,4-dihydroquinolin-2(1h)-one
14. Chebi:31401
15. 6-(4-(1-cyclohexyl-1h-tetrazol-5-yl)butoxy)-3,4-dihydrocarbostyril
16. 3,4-dihydro-6-(4-(1-cyclohexyl-1h-tetrazol-5-yl)butoxy)-2(1h)-quinolinone
17. Nsc-758936
18. 6-[4-(1-cyclohexyl-1h-tetrazol-5-yl)butoxy]-3,4-dihydroquinolin-2(1h)-one
19. Mls000028470
20. 6-[4-(1-cyclohexyl-1h-tetrazol-5-yl)butoxy]-3,4-dihydro-2(1h)-quinolinone
21. 6-[4-(1-cyclohexyl-1h-1,2,3,4-tetrazol-5-yl)butoxy]-1,2,3,4-tetrahydroquinolin-2-one
22. N7z035406b
23. Ncgc00015207-07
24. Smr000058428
25. Dsstox_cid_25132
26. Dsstox_rid_80693
27. Dsstox_gsid_45132
28. 2(1h)-quinolinone, 6-(4-(1-cyclohexyl-1h-tetrazol-5-yl)butoxy)-3,4-dihydro-
29. Cas-73963-72-1
30. Pletal (tn)
31. Sr-01000003107
32. Brn 3632107
33. Unii-n7z035406b
34. 2(1h)-quinolinone, 6-[4-(1-cyclohexyl-1h-tetrazol-5-yl)butoxy]-3,4-dihydro-
35. Cilostazol,(s)
36. Cilostazol-[d11]
37. Mfcd00866780
38. Cilostazol [usan:usp:inn:ban:jan]
39. Tocris-1692
40. Cilostazol [mi]
41. Opera_id_488
42. Cilostazol [inn]
43. Cilostazol [jan]
44. Spectrum2_001118
45. Spectrum3_001170
46. Spectrum4_000772
47. Spectrum5_001762
48. Cilostazol [usan]
49. Lopac-c-0737
50. Cilostazol [vandf]
51. Chembl799
52. 6-[4-(1-cyclohexyl-1h-tetrazol-5-yl)-butoxy]-3,4-dihydro-2(1h)-quinolinone
53. C 0737
54. Cilostazol [mart.]
55. Cilostazol [usp-rs]
56. Cilostazol [who-dd]
57. Lopac0_000218
58. Regid_for_cid_2754
59. Schembl16128
60. Bspbio_002759
61. Kbiogr_001184
62. Mls000758281
63. Mls000759507
64. Mls001076067
65. Mls002153891
66. Spectrum1505230
67. Spbio_001256
68. Cilostazol (jp17/usp/inn)
69. Gtpl7148
70. Cilostazol [orange Book]
71. Dtxsid9045132
72. Hsdb 8312
73. Kbio3_002259
74. Bcpp000279
75. Cilostazol [usp Monograph]
76. Hms1922n15
77. Hms2093m14
78. Hms2096f16
79. Hms2234c06
80. Hms3260l17
81. Hms3268o09
82. Hms3412b18
83. Hms3654j13
84. Hms3676b18
85. Hms3713f16
86. Pharmakon1600-01505230
87. Act02663
88. Bcp03724
89. Zinc1552174
90. Tox21_110098
91. Tox21_500218
92. Bdbm50225508
93. Ccg-39646
94. Nsc758936
95. S1294
96. Akos015855512
97. Cilostazol, >=98% (hplc), Powder
98. Opc 13013; Opc 21; Pletaal
99. Tox21_110098_1
100. Ac-4334
101. Am90304
102. Bcp9000530
103. Cs-1759
104. Db01166
105. Ks-5154
106. Lp00218
107. Nsc 758936
108. Sdccgsbi-0050206.p003
109. 2(1h)-quinolinone, 3,4-dihydro-6-(4-(1-cyclohexyl-1h-tetrazol-5-yl)butoxy)-
110. 2(1h)-quionlinone, 6-(4-(1-cyclohexyl-1h-tetrazol-5-yl)butoxy)-3,4-dihydro-
111. Ncgc00015207-01
112. Ncgc00015207-02
113. Ncgc00015207-03
114. Ncgc00015207-04
115. Ncgc00015207-05
116. Ncgc00015207-06
117. Ncgc00015207-08
118. Ncgc00015207-09
119. Ncgc00015207-10
120. Ncgc00015207-11
121. Ncgc00015207-12
122. Ncgc00015207-25
123. Ncgc00022153-02
124. Ncgc00022153-04
125. Ncgc00022153-05
126. Ncgc00022153-06
127. Ncgc00022153-07
128. Ncgc00260903-01
129. Hy-17464
130. Bcp0726000145
131. Retal;pletal;opc 21;pletaal;cilostal
132. Sbi-0050206.p002
133. Eu-0100218
134. Ft-0602474
135. Ft-0645036
136. Ft-0665038
137. Sw199053-2
138. D01896
139. F20538
140. Ab00382988-14
141. Ab00382988_15
142. Ab00382988_16
143. 963c721
144. A837982
145. Q258591
146. Q-200854
147. Sr-01000003107-2
148. Sr-01000003107-4
149. Sr-01000003107-7
150. Brd-k67017579-001-04-2
151. Brd-k67017579-001-05-9
152. Brd-k67017579-001-07-5
153. Brd-k67017579-001-13-3
154. Brd-k67017579-001-17-4
155. Sr-01000003107-10
156. Cilastatin Sodium, Antibiotic For Culture Media Use Only
157. Cilostazol, United States Pharmacopeia (usp) Reference Standard
158. 6-[4-(1-cyclohexyl-1,2,3,4-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril
159. 6-[4-(l-cyclohexyl-1,2,3,4-tetrazol-5-yl)butoxyl]-3,4-dihydrocarbostyril
160. Cilostazol, Pharmaceutical Secondary Standard; Certified Reference Material
161. 6-(4-(1-cyclohexyl-1h-tetrazol-5-yl)butoxy)quinoline-2,3(1h,4h)-dione
162. 6-[4-(1-cyclohexyl-1h-tetrazol-5-yl)-butoxy]-3,4-dihydro-1h-quinolin-2-one
163. 89332-50-3
| Molecular Weight | 369.5 g/mol |
|---|---|
| Molecular Formula | C20H27N5O2 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 369.21647512 g/mol |
| Monoisotopic Mass | 369.21647512 g/mol |
| Topological Polar Surface Area | 81.9 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 485 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Cilostazol |
| PubMed Health | Cilostazol (By mouth) |
| Drug Classes | Platelet Aggregation Inhibitor |
| Drug Label | Cilostazol is a quinolinone derivative that inhibits cellular phosphodiesterase (more specific for phosphodiesterase III). The empirical formula of cilostazol is C20H27N5O2, and its molecular weight is 369.46. Cilostazol is 6-[4-(1-cyclohexyl-1H-tetr... |
| Active Ingredient | Cilostazol |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 100mg; 50mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Actavis Elizabeth; Breckenridge Pharm; Teva; Apotex; Pliva Hrvatska Doo; Biokey; Sandoz; Mylan; Roxane |
| 2 of 4 | |
|---|---|
| Drug Name | Pletal |
| PubMed Health | Cilostazol (By mouth) |
| Drug Classes | Platelet Aggregation Inhibitor |
| Drug Label | PLETAL (cilostazol) is a quinolinone derivative that inhibits cellular phosphodiesterase (more specific for phosphodiesterase III). The empirical formula of cilostazol is C20H27N5O2, and its molecular weight is 369.46. Cilostazol is 6-[4-(1-cyclohexy... |
| Active Ingredient | Cilostazol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 50mg |
| Market Status | Prescription |
| Company | Otsuka |
| 3 of 4 | |
|---|---|
| Drug Name | Cilostazol |
| PubMed Health | Cilostazol (By mouth) |
| Drug Classes | Platelet Aggregation Inhibitor |
| Drug Label | Cilostazol is a quinolinone derivative that inhibits cellular phosphodiesterase (more specific for phosphodiesterase III). The empirical formula of cilostazol is C20H27N5O2, and its molecular weight is 369.46. Cilostazol is 6-[4-(1-cyclohexyl-1H-tetr... |
| Active Ingredient | Cilostazol |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 100mg; 50mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Actavis Elizabeth; Breckenridge Pharm; Teva; Apotex; Pliva Hrvatska Doo; Biokey; Sandoz; Mylan; Roxane |
| 4 of 4 | |
|---|---|
| Drug Name | Pletal |
| PubMed Health | Cilostazol (By mouth) |
| Drug Classes | Platelet Aggregation Inhibitor |
| Drug Label | PLETAL (cilostazol) is a quinolinone derivative that inhibits cellular phosphodiesterase (more specific for phosphodiesterase III). The empirical formula of cilostazol is C20H27N5O2, and its molecular weight is 369.46. Cilostazol is 6-[4-(1-cyclohexy... |
| Active Ingredient | Cilostazol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 50mg |
| Market Status | Prescription |
| Company | Otsuka |
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Cilostazol is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=cilostazol&Search=Search
Pletal is indicated for the reduction of symptoms of intermittent claudication, as demonstrated by an increased walking distance. /Included in US product label/
NIH; DailyMed. Current Medication Information for Pletal (Cilostazol) Tablet (Updated: July 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24d75b58-bafb-4440-b8d7-4f4079c08b0b
Because of its antiplatelet activity, cilostazol has been used alone or in combination with other antiplatelet agents (e.g., aspirin, clopidogrel) to prevent thrombosis and restenosis following coronary angioplasty/stent implantation. /NOT included in US product label/
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1528
Cilostazol has been used for the secondary prevention of stroke in patients with a history of noncardioembolic stroke or transient ischemic attacks (TIAs). /NOT included in US product label/
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1528
/EXPL THER/ We conducted a randomized, double blind, placebo controlled trial to assess the efficacy and safety of cilostazol, a selective inhibitor of phosphodiesterase 3, in patients with vasospastic angina (VSA). Cilostazol has been shown to induce vascular dilatation, but its efficacy in patients with VSA is unknown. Between October 2011 and July 2012, 50 patients with confirmed VSA who had >/= 1 angina episodes/week despite amlodipine therapy (5 mg/day) were randomly assigned to receive either cilostazol (up to 200 mg/day) or placebo for 4 weeks. All patients were given diaries to record the frequency and severity of chest pain (0-10 grading). The primary endpoint was the relative reduction of the weekly incidence of chest pain. Baseline characteristics were similar between the two groups. Among 49 evaluable patients (25 in the cilostazol group, 24 in the placebo group), the primary endpoint was significantly greater in the cilostazol group compared with the placebo group (-66.5 +/- 88.6% vs -17.6 +/- 140.1%, respectively, p=0.009). The secondary endpoints, including a change in the frequency of chest pain (-3.7 +/- 0.5 vs -1.9 +/- 0.6, respectively, p=0.029), a change in the chest pain severity scale (-2.8 +/- 0.4 vs -1.1 +/- 0.4, respectively, p=0.003), and the proportion of chest pain-free patients (76.0% vs 33.3%, respectively, p=0.003) also significantly favoured cilostazol. Headache was the most common adverse event in both groups (40.0% vs 20.8%, respectively, p=0.217). Cilostazol is an effective therapy for patients with VSA uncontrolled by conventional amlodipine therapy, and has no serious side effects.
PMID:24934484 Shin ES et al; Heart 100 (19): 1531-6 (2014)
/BOXED WARNING/ WARNING: CONTRAINDICATED IN HEART FAILURE PATIENTS. Pletal is contraindicated in patients with heart failure of any severity. Cilostazol and several of its metabolites are inhibitors of phosphodiesterase III. Several drugs with this pharmacologic effect have caused decreased survival compared to placebo in patients with class III-IV heart failure.
NIH; DailyMed. Current Medication Information for Pletal (Cilostazol) Tablet (Updated: July 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24d75b58-bafb-4440-b8d7-4f4079c08b0b
Rare cases of thrombocytopenia or leukopenia progressing to agranulocytosis have been reported when cilostazol was not immediately discontinued; agranulocytosis was reversible with discontinuance of cilostazol.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1529
Information is limited regarding the safety and efficacy of concurrent use of cilostazol and clopidogrel. Currently it is unknown whether concurrent therapy with cilostazol and clopidogrel has additive effects on bleeding time. Caution should be used and bleeding times monitored during such concurrent therapy.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1529
Cilostazol may induce tachycardia, palpitation, tachyarrhythmia or hypotension. The increase in heart rate associated with cilostazol is approximately 5 to 7 bpm. Patients with a history of ischemic heart disease may be at risk for exacerbations of angina pectoris or myocardial infarction.
NIH; DailyMed. Current Medication Information for Pletal (Cilostazol) Tablet (Updated: July 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24d75b58-bafb-4440-b8d7-4f4079c08b0b
For more Drug Warnings (Complete) data for Cilostazol (10 total), please visit the HSDB record page.
Indicated for the alleviation of symptoms of intermittent claudication (pain in the legs that occurs with walking and disappears with rest).
Cilostazol reduces the symptoms of intermittent claudication, as indicated by an increased walking distance. Intermittent claudication is pain in the legs that occurs with walking and disappears with rest. The pain occurs due to reduced blood flow to the legs.
Fibrinolytic Agents
Fibrinolysin or agents that convert plasminogen to FIBRINOLYSIN. (See all compounds classified as Fibrinolytic Agents.)
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Phosphodiesterase 3 Inhibitors
Compounds that specifically inhibit PHOSPHODIESTERASE 3. (See all compounds classified as Phosphodiesterase 3 Inhibitors.)
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AC - Platelet aggregation inhibitors excl. heparin
B01AC23 - Cilostazol
Absorption
Cilostazol is absorbed after oral administration. A high fat meal increases absorption, with an approximately 90% increase in Cmax and a 25% increase in AUC. Absolute bioavailability is not known.
Route of Elimination
Cilostazol is extensively metabolized by hepatic cytochrome P-450 enzymes, mainly 3A4, and, to a lesser extent, 2C19, with metabolites largely excreted in urine. Cilostazol is eliminated predominately by metabolism and subsequent urinary excretion of metabolites. The primary route of elimination was via the urine (74%), with the remainder excreted in feces (20%). No measurable amount of unchanged cilostazol was excreted in the urine, and less than 2% of the dose was excreted as 3,4-dehydro-cilostazol. About 30% of the dose was excreted in urine as 4'-trans-hydroxy-cilostazol.
/MILK/ Transfer of cilostazol into milk has been reported in rats.
NIH; DailyMed. Current Medication Information for Pletal (Cilostazol) Tablet (Updated: July 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24d75b58-bafb-4440-b8d7-4f4079c08b0b
Following oral administration of a single 100-mg dose of cilostazol with a high-fat meal, peak plasma cilostazol concentrations and area under the plasma concentration-time curve (AUC) increased by approximately 90 and 25%, respectively.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1529
Pletal is absorbed after oral administration. A high fat meal increases absorption, with an approximately 90% increase in Cmax and a 25% increase in AUC. Absolute bioavailability is not known.
NIH; DailyMed. Current Medication Information for Pletal (Cilostazol) Tablet (Updated: July 2015). Available from, as of January 25, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24d75b58-bafb-4440-b8d7-4f4079c08b0b
The primary route of elimination was via the urine (74%), with the remainder excreted in feces (20%). No measurable amount of unchanged cilostazol was excreted in the urine, and less than 2% of the dose was excreted as 3,4-dehydro-cilostazol. About 30% of the dose was excreted in urine as 4'-trans-hydroxy-cilostazol. The remainder was excreted as other metabolites, none of which exceeded 5%. There was no evidence of induction of /microsomal enzymes/.
NIH; DailyMed. Current Medication Information for Pletal (Cilostazol) Tablet (Updated: July 2015). Available from, as of January 25, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24d75b58-bafb-4440-b8d7-4f4079c08b0b
For more Absorption, Distribution and Excretion (Complete) data for Cilostazol (7 total), please visit the HSDB record page.
Hepatic. Cilostazol is extensively metabolized by hepatic cytochrome P-450 enzymes, mainly 3A4, and, to a lesser extent, 2C19, with metabolites largely excreted in urine. Two metabolites are active, with one metabolite appearing to account for at least 50% of the pharmacologic (PDE III inhibition) activity after administration of cilostazol.
Following oral administration of 100 mg radiolabeled cilostazol, 56% of the total analytes in plasma was cilostazol, 15% was 3,4-dehydro-cilostazol (4-7 times as active as cilostazol), and 4% was 4'-trans-hydroxy-cilostazol (20% as active as cilostazol).
NIH; DailyMed. Current Medication Information for Pletal (Cilostazol) Tablet (Updated: July 2015). Available from, as of January 25, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24d75b58-bafb-4440-b8d7-4f4079c08b0b
Cilostazol is eliminated predominantly by metabolism and subsequent urinary excretion of metabolites. Based on in vitro studies, the primary isoenzymes involved in cilostazol's metabolism are CYP3A4 and, to a lesser extent, CYP2C19. The enzyme responsible for metabolism of 3,4-dehydro-cilostazol, the most active of the metabolites, is unknown.
NIH; DailyMed. Current Medication Information for Pletal (Cilostazol) Tablet (Updated: July 2015). Available from, as of January 25, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24d75b58-bafb-4440-b8d7-4f4079c08b0b
Cilostazol is extensively metabolized by hepatic cytochrome P-450 enzymes, mainly 3A4, and, to a lesser extent, 2C19, with metabolites largely excreted in urine. Two metabolites are active, with one metabolite appearing to account for at least 50% of the pharmacologic (PDE III inhibition) activity after administration of Pletal.
NIH; DailyMed. Current Medication Information for Pletal (Cilostazol) Tablet (Updated: July 2015). Available from, as of January 25, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24d75b58-bafb-4440-b8d7-4f4079c08b0b
The pharmacokinetics of cilostazol was investigated after oral and intravenous administration in both male and female rats. After oral administration, area under serum concentration-time curve (AUC) was about 35-fold higher in female rats than in male rats, and absolute bioavailability was about 5.8-fold higher in female rats than in male rats. Total body clearance (CL(total)) for female rats was around one-sixth of that for male rats. In vivo hepatic clearance (CL(h)) calculated based on isolated liver perfusion studies was even higher than or around 90% of the in vivo CL(total) of cilostazol for female and male rats, respectively, indicating that cilostazol is mainly eliminated by the liver in both male and female rats. In vitro metabolism studies utilizing hepatic microsomes and recombinant cytochrome (CYP) isoforms clearly indicated that major metabolites of cilostazol were generated extensively with hepatic microsomes of male rats and that male-predominant CYP3A2 and male-specific CYP2C11 were mainly responsible for the hepatic metabolism of cilostazol. Therefore, the great sex differences in the pharmacokinetics of cilostazol were mainly attributed to the large difference in hepatic metabolism. Our experimental results also suggested that the substantial metabolism of cilostazol in the small intestine and its possible saturation would be responsible for dose-dependent bioavailability in both male and female rats.
PMID:21718207 Kamada N et al; Xenobiotica 41 (10): 903-13 (2011)
The primary route of elimination was via the urine (74%), with the remainder excreted in feces (20%). No measurable amount of unchanged cilostazol was excreted in the urine, and less than 2% of the dose was excreted as 3,4-dehydro-cilostazol. About 30% of the dose was excreted in urine as 4'-trans-hydroxy-cilostazol. The remainder was excreted as other metabolites, none of which exceeded 5%. There was no evidence of induction of /microsomal enzymes/.
NIH; DailyMed. Current Medication Information for Pletal (Cilostazol) Tablet (Updated: July 2015). Available from, as of January 25, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24d75b58-bafb-4440-b8d7-4f4079c08b0b
Cilostazol has known human metabolites that include OPC-13217 and OPC-13326.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
11-13 hours.
Cilostazol and its active metabolites have apparent elimination half-lives of about 11-13 hours.
NIH; DailyMed. Current Medication Information for Pletal (Cilostazol) Tablet (Updated: July 2015). Available from, as of January 25, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24d75b58-bafb-4440-b8d7-4f4079c08b0b
Cilostazol and several of its metabolites are cyclic AMP (cAMP) phosphodiesterase III inhibitors (PDE III inhibitors), inhibiting phosphodiesterase activity and suppressing cAMP degradation with a resultant increase in cAMP in platelets and blood vessels, leading to inhibition of platelet aggregation and vasodilation.
Cilostazol, a phosphodiesterase 3, has been widely used in patients with arterial disease and is known to have additional beneficial effects on dyslipidemia. However, the effect of cilostazol on hepatic steatosis has not been fully elucidated. We investigated the effect of cilostazol on hepatic ABCA1 expression and hepatic steatosis in diet-induced obesity mice model. Hepatic ABCA1 expression and lipid accumulation were analyzed in HepG2 cell lines treated with cilostazol. Male C57BL/6 mice were randomly divided into three groups: (1) fed normal chow diet with vehicle; (2) fed high-fat diet (HFD) with vehicle; (3) fed HFD with cilostazol. Cilostazol (30 mg/kg) was orally administered once daily for 9 weeks. Cilostazol significantly enhanced ABCA1 expression and restored ABCA1 expression reduced by palmitate in HepG2 cells. Cilostazol treatment ameliorated lipid accumulation induced by palmitate, and this effect was diminished when ABCA1 or LRP1 was silenced by small interference RNA. After silencing of LRP1, ABCA1 expression was decreased in HepG2 cells. Cilostazol significantly enhanced hepatic ABCA1 expression and decreased hepatic fat in HFD-fed mice. Hepatic expression of cleaved caspase-3 and PARP1 was also decreased in HFD-fed mice treated with cilostazol. Cilostazol ameliorated hepatic steatosis and increased ABCA1 expression in the hepatocytes. Enhancing ABCA1 expression with cilostazol represents a potential therapeutic avenue for treatment of hepatic steatosis.
PMID:26362727 Jeon BH et al; Metabolism 64 (11): 1444-53 (2015)
Cilostazol, a quinolinone-derivative selective phosphodiesterase (PDE) inhibitor, is a platelet-aggregation inhibitor and arterial vasodilator. Although the mechanism of action of cilostazol has not been fully elucidated, the drug appears to inhibit activation of cellular PDE type III (PDE III), resulting in suppressed degradation, and thus increased concentrations, of cyclic adenosine-3',5'-monophosphate (cAMP) in platelets and blood vessels. Increased cAMP concentrations are thought to result in arterial vasodilation and inhibition of platelet aggregation.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1529

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?