
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
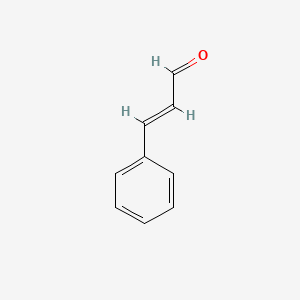

1. 3-phenyl-2-propenal
2. 3-phenylprop-2-enaldehyde
3. Beta-phenylacrolein
4. Cinnamic Aldehyde
5. Cinnamic Aldehyde, (e)-isomer
6. Supercinnamaldehyde
7. Trans-3-phenylprop-2-enaldehyde
8. Trans-cinnamaldehyde
1. Trans-cinnamaldehyde
2. 104-55-2
3. 14371-10-9
4. Cinnamic Aldehyde
5. 3-phenylacrylaldehyde
6. Cinnamal
7. (e)-cinnamaldehyde
8. Zimtaldehyde
9. 2-propenal, 3-phenyl-
10. 3-phenylpropenal
11. Phenylacrolein
12. (2e)-3-phenylprop-2-enal
13. (e)-3-phenylprop-2-enal
14. Trans-cinnamic Aldehyde
15. Cinnamylaldehyde
16. (e)-3-phenylpropenal
17. (e)-3-phenyl-2-propenal
18. Cassia Aldehyde
19. 3-phenylacrolein
20. 3-phenyl-2-propenal
21. Cinnemaldehyde
22. 2-propenal, 3-phenyl-, (2e)-
23. Cinnamyl Aldehyde
24. Abion Ca
25. Cinnamaldehyde, (e)-
26. Trans-cinnamylaldehyde
27. Benzylideneacetaldehyde
28. Beta-phenylcrolein
29. 3-phenyl-2-propenaldehyde
30. Acrolein, 3-phenyl-
31. 3-fenylpropenal
32. Fema No. 2286
33. 3-phenyl-2-propen-1-al
34. Trans-3-phenyl-2-propenal
35. (e)-cinnamic Aldehyde
36. 3-phenylprop-2-enal
37. Nci-c56111
38. 2-propenal, 3-phenyl-, (e)-
39. (2e)-3-phenylacrylaldehyde
40. .beta.-phenylacrolein
41. Chebi:16731
42. Cinnamaldehyde, Trans-
43. (e)-3-phenyl-propenal
44. Mfcd00007000
45. Nsc-16935
46. Nsc-40346
47. Sr60a3xg0f
48. (3e)-3-phenylprop-2-enal
49. (2e)-3-phenyl-2-propenal
50. Chembl293492
51. Aldehyd Skoricovy
52. Xc-800
53. Ncgc00091512-04
54. Nsc 16935
55. Dsstox_cid_4834
56. Wln: Vh1u1r
57. Dsstox_rid_77548
58. Dsstox_gsid_24834
59. Caswell No. 221a
60. Fema Number 2286
61. 3-fenylpropenal [czech]
62. Aldehyd Skoricovy [czech]
63. Cinnamic Aldehyde (natural)
64. Hefty Dog And Cat Repellent
65. Cas-14371-10-9
66. Ccris 3189
67. Ccris 6222
68. Cinnamaldehyde [nf]
69. Hsdb 209
70. Einecs 203-213-9
71. Unii-sr60a3xg0f
72. Epa Pesticide Chemical Code 040506
73. Brn 0605737
74. Brn 1071571
75. E-cinnamaldehyde
76. Ai3-00473
77. Ai3-33275
78. Transcinnamaldehyde
79. Trans Cinnamaldehyde
80. Nat. Cinnamaldehyde
81. Cnma
82. (trans)-cinnamaldehyde
83. Trans Cinnamic Aldehyde
84. Trans-3-phenylacrolein
85. Cinnamaldehyde, Natural
86. Cinnamal [inci]
87. Trans-3-phenyl-propenal
88. (e)-phenylvinyl Aldehyde
89. (e)-3-phenylacrylaldehyde
90. Bmse010257
91. Epitope Id:150921
92. Cinnamaldehyde [ii]
93. Cinnamaldehyde [mi]
94. Ec 203-213-9
95. Trans-3-phenylacrylaldehyde
96. Trans-cinnamaldehyde ,(s)
97. Schembl3441
98. Trans-cinnamaldehyde, 99%
99. (e)-3-phenyl-acrylaldehyde
100. Cinnamaldehyde [fcc]
101. Trans-cinnamaldehyde; Trans-3-phenylacrylaldehyde
102. Cinnamaldehyde (trans), Neat
103. Cinnamaldehyde [fhfi]
104. Cinnamaldehyde [hsdb]
105. 2-07-00-00273 (beilstein Handbook Reference)
106. 4-07-00-00984 (beilstein Handbook Reference)
107. Mls002454394
108. Cinnamaldehyde (trans)
109. Trans-cinnamaldehyde, >=99%
110. Gtpl2423
111. (e)-cinnamaldehyde (incorrect)
112. Cinnamaldehyde [usp-rs]
113. Cinnamaldehyde [who-dd]
114. Natural Cinnamic Aldehyde
115. Dtxsid6024834
116. Trans-cinnamaldehyde (incorrect)
117. Chebi:142921
118. Hms2268o08
119. Hms3885e04
120. Hy-n0609
121. Nsc16935
122. Nsc40346
123. Zinc1532777
124. Tox21_111144
125. Tox21_201804
126. Tox21_303271
127. Bdbm50203065
128. S3763
129. Stk397371
130. Akos000119171
131. Cinnamaldehyde, Natural, >=95%, Fg
132. Ccg-266119
133. Db14184
134. Cinnamaldehyde Min. 98%, For Synthesis
135. Cinnamaldehyde 100 Microg/ml In Toluene
136. Ncgc00091512-01
137. Ncgc00091512-02
138. Ncgc00091512-05
139. Ncgc00091512-06
140. Ncgc00091512-07
141. Ncgc00257017-01
142. Ncgc00259353-01
143. Trans-cinnamaldehyde, Analytical Standard
144. As-12078
145. As-75456
146. Smr000112334
147. Trans-cinnamaldehyde, >=98%, Fcc, Fg
148. (e)-3-phenyl-2-propenal(e)-cinnamaldehyde
149. Db-003796
150. Am20060482
151. Cs-0009609
152. N1482
153. A14480
154. C00903
155. Cinnamaldehyde, Vetec(tm) Reagent Grade, 93%
156. D72477
157. A801001
158. Q204036
159. W-205597
160. B99dd6c7-1c6d-4fe3-a172-54bfdb987683
161. Cinnamaldehyde (constituent Of Cinnamomum Verum Bark) [dsc]
162. Cinnamaldehyde, United States Pharmacopeia (usp) Reference Standard
163. Trans-cinnamaldehyde (constituent Of Cinnamomum Cassia Bark) [dsc]
| Molecular Weight | 132.16 g/mol |
|---|---|
| Molecular Formula | C9H8O |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 132.057514874 g/mol |
| Monoisotopic Mass | 132.057514874 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 121 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Cinnamonum zeylanicum (cinnamon) is widely used in traditional system of medicine to treat diabetes in India. The present study was carried out to isolate and identify the putative antidiabetic compounds ... Cinnamaldehyde was administered at different doses (5, 10 and 20 mg/kg bw) for 45 days to streptozotocin (STZ) (60 mg/kg bw)-induced male diabetic wistar rats. It was found that plasma glucose concentration was significantly (p<0.05) decreased in a dose-dependent manner (63.29%) compared to the control. In addition, oral administration of cinnamaldehyde (20 mg/kg bw) significantly decreased glycosylated hemoglobin (HbA(1C)), serum total cholesterol, triglyceride levels and at the same time markedly increased plasma insulin, hepatic glycogen and high-density lipoprotein-cholesterol levels. Also cinnamaldehyde restored the altered plasma enzyme (aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, alkaline phosphatase and acid phosphatase) levels to near normal. Administration of glibenclamide, a reference drug (0.6 mg/kg bw) also produced a significant (p < 0.05) reduction in blood glucose concentration in STZ-induced diabetic rats. The results of this experimental study indicate that cinnamaldehyde possesses hypoglycemic and hypolipidemic effects in STZ-induced diabetic rats.
PMID:17140783 Subash BP et al; Phytomedicine 14 (1): 15-22 (2007).
The probable oral lethal dose for humans is 0.5 to 5 g/kg for a 70-kg person. Both the oil and pure aldehyde are irritants, especially if undiluted. They can also cause inflammation and erosion of gastrointestinal mucosa. Prolonged skin contact (more than 48 hr) can produce superficial partial thickness burns
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V5 1048
3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG, BETWEEN 1 OZ & 1PINT (OR 1 LB) FOR 70 KG PERSON (150 LB).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-256
Cinnamaldehyde is approved by the FDA for use within allergenic epicutaneous patch tests which are indicated for use as an aid in the diagnosis of allergic contact dermatitis (ACD) in persons 6 years of age and older.
Antimutagenic Agents
Agents that reduce the frequency or rate of spontaneous or induced mutations independently of the mechanism involved. (See all compounds classified as Antimutagenic Agents.)
Flavoring Agents
Substances added to foods and medicine to improve the taste. (See all compounds classified as Flavoring Agents.)
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)
Absorption
Cinnamaldehyde is 52% absorbed through the skin and shown to be rapidly absorbed from the gut.
Route of Elimination
Cinnamaldehyde is metabolized and excreted primarily in the urine and, to a minor extent, in the feces. After oral or intraperitoneal administration to rats and mice, 6998% of the dose of cinnamaldehyde was recovered in the urine and feces within 24 h.
The bioavailability of microencapsulated cinnamaldehyde (CNMA) was investigated in male F344 rats. Rats were gavaged with CNMA in corn oil using either microencapsulated or the neat chemical at doses of 50, 250, and 500 mg/kg. No differences between the two formulations at any of the doses were found in either CNMA blood concentration profiles or in the rate of urinary hippuric acid excretion. Both formulations showed a low bioavailability (< 20%) at 250 and 500 mg/kg. Regardless of the formulation used, oral gavage of CNMA significantly increased the urinary excretion of hippuric acid. About 75% of the dose of CNMA was metabolized to hippuric acid and recovered in the urine. The total amount of hippuric acid recovered in a 50-hr urinary collection correlated well with the CNMA dose. The data suggest that there was complete release of CNMA from the microcapsules and that microencapsulation of CNMA does not affect its bioavailability or its metabolism ...
PMID:8432430 Yuan J et al; Fundam Appl Toxicol 20 (1): 83-7 (1993).
/Cinnamaldehyde is/ presumably oxidized in vivo to cinnamic acid, which is excreted in urine as benzoic and hippuric acids.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-256
After ip admin of cinnamic aldehyde to rats, urinary thio ether excretion amounted to 6.5% of dose.
DELBRESSINE LP C ET AL; BR J PHARMACOL 68 (1): 165 (1980)
Cinnamaldehyde administered intraperitoneally to a rabbit was excreted in the urine as cinnamic acid, cinnamoylglycine, benzoic acid and hippuric acid.
WHO/FAO; Joint Expert Committee on Food Additives (JECFA) - Monographs and Evaluations; Food Additive Series 14: Cinnamaldehyde (1981). Available from database query, as of July 13, 2009: https://www.inchem.org/pages/jecfa.html
For more Absorption, Distribution and Excretion (Complete) data for CINNAMALDEHYDE (7 total), please visit the HSDB record page.
The metabolism of trans-[3-14C]cinnamaldehyde was investigated in male and female Fischer 344 rats and CD1 mice at doses of 2 and 250 mg/kg bw given by ip injection and in males at 250 mg/kg by oral gavage. Some 94% of the administered dose was recovered in the excreta in 72 hr in both species with most (75-81%) present in the 0-24-hr urine. Less than 2% of the administered dose was found in the carcasses at 72 hr after dosing. Urinary metabolites were identified by their chromatographic characteristics. In both species the major urinary metabolite was hippuric acid accompanied by 3-hydroxy-3-phenylpropionic acid, benzoic acid and benzoyl glucuronide. The glycine conjugate of cinnamic acid was formed to a considerable extent only in the mouse. The oxidative metabolism of cinnamaldehyde essentially follows that of cinnamic acid, by beta-oxidation analogous to that of fatty acids. Apart from the metabolites common to cinnamic acid and cinnamaldehyde, 7% of 0-24-hr urinary 14C was accounted for by two new metabolites in the rat and three in the mouse, which have been shown in other work to arise from a second pathway of cinnamaldehyde metabolism involving conjugation with glutathione. The excretion pattern and metabolic profile of cinnamaldehyde in rats and mice are not systematically affected by sex, dose size and route of administration. The data are discussed in terms of their relevance to the safety evaluation of trans-cinnamaldehyde, particularly the validity or otherwise of extrapolation of toxicity data from high to low dose. /trans-Cinnamaldehyde/
PMID:7959441 Peters MM, Caldwell J; Food Chem Toxicol 32 (10): 869-76 (1994).
To evaluate the extent of cinnamaldehyde and cinnamic alcohol metabolism in human skin and provide evidence for the role of cutaneous alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) in such metabolism ... the extent of cinnamic alcohol and aldehyde metabolism was investigated in human skin homogenates and sub-cellular fractions ... Studies were conducted in the presence and absence of the ADH/cytochrome P450 inhibitor 4-methylpyrazole and the cytosolic ALDH inhibitor, disulfiram. Differential metabolism of cinnamic alcohol and cinnamaldehyde was observed in various subcellular fractions: skin cytosol was seen to be the major site of cinnamic compound metabolism. Significant metabolic inhibition was observed using 4-methylpyrazole and disulfiram in whole skin homogenates and cytosolic fractions only ... This study has demonstrated that cutaneous ADH and ALDH activities, located within defined subcellular compartments, play important roles in the activation and detoxification of CAlc and CAld in skin ...
PMID:12615359 Cheung C et al; J Dermatol Sci 31 (1): 9-19 (2003).
Cinnamaldehyde administered intraperitoneally to a rabbit was excreted in the urine as cinnamic acid, cinnamoylglycine, benzoic acid and hippuric acid.
WHO/FAO; Joint Expert Committee on Food Additives (JECFA) - Monographs and Evaluations; Food Additive Series 14: Cinnamaldehyde (1981). Available from database query, as of July 13, 2009: https://www.inchem.org/pages/jecfa.html
Identification of 2 sulfur containing urinary metabolites of cinnamic aldehyde in rat which are 3-S-(N-acetylcysteinyl)-3-phenylpropyl alcohol and 3-S-(N-acetylcysteinyl)-3-phenylpropionic acid.
DELBRESSINE LP C ET AL; BR J PHARMACOL 68 (1): 165 (1980)
For more Metabolism/Metabolites (Complete) data for CINNAMALDEHYDE (6 total), please visit the HSDB record page.
Cinnamaldehyde is a known human metabolite of cinnarizine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
55
PharmaCompass offers a list of Cinnamaldehyde API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Cinnamaldehyde manufacturer or Cinnamaldehyde supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Cinnamaldehyde manufacturer or Cinnamaldehyde supplier.
PharmaCompass also assists you with knowing the Cinnamaldehyde API Price utilized in the formulation of products. Cinnamaldehyde API Price is not always fixed or binding as the Cinnamaldehyde Price is obtained through a variety of data sources. The Cinnamaldehyde Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Cinnamaldehyde manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Cinnamaldehyde, including repackagers and relabelers. The FDA regulates Cinnamaldehyde manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Cinnamaldehyde API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Cinnamaldehyde supplier is an individual or a company that provides Cinnamaldehyde active pharmaceutical ingredient (API) or Cinnamaldehyde finished formulations upon request. The Cinnamaldehyde suppliers may include Cinnamaldehyde API manufacturers, exporters, distributors and traders.
click here to find a list of Cinnamaldehyde suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Cinnamaldehyde DMF (Drug Master File) is a document detailing the whole manufacturing process of Cinnamaldehyde active pharmaceutical ingredient (API) in detail. Different forms of Cinnamaldehyde DMFs exist exist since differing nations have different regulations, such as Cinnamaldehyde USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Cinnamaldehyde DMF submitted to regulatory agencies in the US is known as a USDMF. Cinnamaldehyde USDMF includes data on Cinnamaldehyde's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Cinnamaldehyde USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Cinnamaldehyde suppliers with USDMF on PharmaCompass.
Cinnamaldehyde Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Cinnamaldehyde GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Cinnamaldehyde GMP manufacturer or Cinnamaldehyde GMP API supplier for your needs.
A Cinnamaldehyde CoA (Certificate of Analysis) is a formal document that attests to Cinnamaldehyde's compliance with Cinnamaldehyde specifications and serves as a tool for batch-level quality control.
Cinnamaldehyde CoA mostly includes findings from lab analyses of a specific batch. For each Cinnamaldehyde CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Cinnamaldehyde may be tested according to a variety of international standards, such as European Pharmacopoeia (Cinnamaldehyde EP), Cinnamaldehyde JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Cinnamaldehyde USP).
