
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
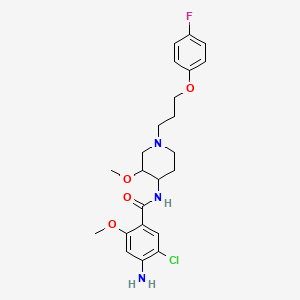

1. Propulsid
2. R 51619
3. R-51619
4. R51619
1. Prepulsid
2. Propulsid
3. 81098-60-4
4. 104860-73-3
5. 4-amino-5-chloro-n-[1-[3-(4-fluorophenoxy)propyl]-3-methoxypiperidin-4-yl]-2-methoxybenzamide
6. Risamal
7. 4-amino-5-chloro-n-{1-[3-(4-fluorophenoxy)propyl]-3-methoxy-4-piperidyl}-2-methoxybenzamide
8. T 1341
9. Dsstox_cid_2825
10. 4-amino-5-chloro-n-{1-[3-(4-fluorophenoxy)propyl]-3-methoxypiperidin-4-yl}-2-methoxybenzamide
11. Dsstox_rid_76746
12. Dsstox_gsid_22825
13. Ncgc00016944-01
14. 4-amino-5-chloro-n-(1-(3-(4-fluorophenoxy)propyl)-3-methoxypiperidin-4-yl)-2-methoxybenzamide
15. Benzamide, 4-amino-5-chloro-n-(1-(3-(4-fluorophenoxy)propyl)-3-methoxy-4-piperidinyl)-2-methoxy-, Cis-
16. Benzamide, 4-amino-5-chloro-n-[1-[3-(4-fluorophenoxy)propyl]-3-methoxy-4-piperidinyl]-2-methoxy-, Cis-
17. Prestwick_786
18. Cas-81098-60-4
19. Cisapride (usp/inn)
20. Cisapride-[13c,d3]
21. (.+/-.)-cisapride
22. Prestwick0_000430
23. Prestwick1_000430
24. Prestwick2_000430
25. Chembl1729
26. Schembl16131
27. Gtpl240
28. Spbio_002359
29. Chebi:95129
30. Cid_5311047
31. Hms1569e22
32. Hms3370f16
33. 4-amino-5-chloro-n-[1-[3-(4-fluorophenoxy)propyl]-3-methoxy-4-piperidinyl]-2-methoxybenzamide
34. 4-amino-5-chloro-n-{1-[3-(4-fluoro-phenoxy)-propyl]-3-methoxy-piperidin-4-yl}-2-methoxy-benzamide
35. Tox21_110699
36. Bdbm50005836
37. Stl058624
38. Akos005710823
39. Tox21_110699_1
40. Ac-1912
41. Ccg-213491
42. Ncgc00025262-02
43. Ncgc00168465-01
44. 4-amino-5-chloro-n-[1-{3-[(4-fluorophenyl)oxy]propyl}-3-(methyloxy)piperidin-4-yl]-2-(methyloxy)benzamide
45. Ls-15011
46. Ft-0623916
47. C06910
48. D00274
49. L000938
50. Brd-a12896037-001-02-7
51. 4-amino-5-chloro-2-methoxy-n-[1-[3-(4-fluorophenoxy)propyl]-3-methoxy-4-piperidinyl]benzamide
52. 4-amino-5-chloro-n-(1-[3-(4-fluorophenoxy)propyl]-3-methoxy-4-piperidinyl)-2-methoxybenzamide, Cis- #
| Molecular Weight | 465.9 g/mol |
|---|---|
| Molecular Formula | C23H29ClFN3O4 |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 9 |
| Exact Mass | 465.1830623 g/mol |
| Monoisotopic Mass | 465.1830623 g/mol |
| Topological Polar Surface Area | 86 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 581 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Ulcer Agents; Gastrointestinal Agents; Serotonin Agonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Cisapride is indicated for the symptomatic treatment of nocturnal (and daytime /Not included in US product labeling/) heartburn, and of esophagitis due to reflux and delayed gastric emptying. Treatment may continue for up to 8 weeks; however, tolerance to cisapride may develop at some point in therapy. /Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 849
Cisapride is indicated in the treatment of gastroparesis, including idiopathic, diabetic, and intestinal pseudo-obstruction. Treatment may continue for up to 8 weeks; however, tolerance to cisapride may develop at some point in therapy. /NOT included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 849
... Reduce the consumption of laxatives in patients who chronically abuse these agents.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 946
For more Therapeutic Uses (Complete) data for CISAPRIDE (7 total), please visit the HSDB record page.
Cisapride generally is well tolerated. Adverse effects on the GI tract and nervous system are most common and those most frequently requiring discontinuance of the drug(usually because of intolerable diarrhea and/or abdominal pain). The most common adverse GI effects (e.g., diarrhea) are extensions of the drug's pharmacologic activity. Because of differences in the pharmacologic profiles of the drugs, adverse nervous system effects are less common with cisapride than with metoclopramide whereas diarrhea is more common with cisapride.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2146
In adults receiving cisapride for motility disorder in US placebo-controlled clinical trials, including those with gastroesophageal reflux disease, the most frequent adverse effects of cisapride were headache, diarrhea, abdominal pain, nausea, constipation, and rhinitis. The frequency of diarrhea, abdominal pain, constipation, flatulence, and rhinitis appears to be dose dependent, occurring more frequently in patients receiving oral cisapride 20 mg 4 times daily than in those receiving 10 mg 4 times daily. Many adverse effects reported with cisapride occurred at a frequency similar to that associated with placebo, and a causal relationship to the drug often could not be established.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2146
Dehydration was reported in more than 1% of patients receiving cisapride in controlled clinical trials. Limited evidence indicates that cisapride does not adversely affect glycemic control in insulin-dependent (type I) diabetic patients with delayed gastric emptying.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2147
Viral infection occurred in about 4% of patients receiving cisapride in controlled clinical trails and required discontinuance of the drug in 0.2% of patients. Fever was reported in about 2% of patients receiving cisapride in controlled clinical trials and required discontinuance in 0.1%.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2147
For more Drug Warnings (Complete) data for CISAPRIDE (6 total), please visit the HSDB record page.
Serotonin Receptor Agonists
Endogenous compounds and drugs that bind to and activate SEROTONIN RECEPTORS. Many serotonin receptor agonists are used as ANTIDEPRESSANTS; ANXIOLYTICS; and in the treatment of MIGRAINE DISORDERS. (See all compounds classified as Serotonin Receptor Agonists.)
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)
A - Alimentary tract and metabolism
A03 - Drugs for functional gastrointestinal disorders
A03F - Propulsives
A03FA - Propulsives
A03FA02 - Cisapride
The placental transfer of cisapride, a new prokinetic agent, was studied in a sheep model. The pharmacokinetics of cisapride were studied in the lamb, the pregnant ewe, and the fetus by obtaining blood samples from chronically implanted arterial catheters. Comparable pharmacokinetic parameters were found in the lamb and the adult sheep: half-life, 1.39-1.83 hr; total plasma clearance, 1998-2160 ml/kg/hr; AUC, 92.6-100.1 ng.hr/ml. Cisapride plasma concentrations after continuous infusion were predicted correctly based on the parameters obtained after IV bolus. There was a materno-fetal transfer of cisapride following a single IV bolus administered to the mother. Cisapride crossed the placenta within 5 min and equilibrated with maternal plasma within 20 to 30 min after dosing. The average fetal-to-maternal plasma concentration ratio was 0.71. The amniotic fluid also contained measurable amounts of cisapride. The protein binding of cisapride in maternal and fetal plasma is 89.0% and 88.4%, respectively; the free fraction is 4 times larger than in humans. Cisapride crosses the ovine placental barrier. The sheep placenta is less permeable than the human placenta, but the higher free fraction of cisapride facilitates placental transfer.
PMID:1673393 Veereman-Wauters G et al; Drug Metab Dispos 19 (1): 168-72 (1991)
IPA COPYRIGHT: ASHP The metabolism of cisapride in vitro using Liver fractions of dogs, rabbits, and rats and the metabolites identified by high performance LC and by MS are described. Main bi otransformat i on routes were oxi dat i ve N-dealkylat i on at the pi peri di ne ni trogen and aron at i c hydroxylat i on at the fluorophenyl or at the benzami de moi ety. ENG ~21 nq~_~n_~.
(+-)-Cisapride has known human metabolites that include 3-Fluoro-4-hydroxycisapride, 4-Fluoro-2-hydroxycisapride, and Norcisapride.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Cisapride exerts its effect by increasing the release of acetylcholine from the postganglionic nerve endings of the myenteric plexus. This release of acetylcholine increases esophageal activity and increases esophageal sphincter tone, thereby improving esophageal clearance and decreasing reflux of gastric and duodenal emptying as a result of increased gastric and duodenal contractility and antroduodenal coordination. Duodenogastric reflux is also decreased. Cisapride improves transit in both small and large bowel.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 849

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
31
PharmaCompass offers a list of Cisapride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Cisapride manufacturer or Cisapride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Cisapride manufacturer or Cisapride supplier.
PharmaCompass also assists you with knowing the Cisapride API Price utilized in the formulation of products. Cisapride API Price is not always fixed or binding as the Cisapride Price is obtained through a variety of data sources. The Cisapride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Cisapride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Cisapride, including repackagers and relabelers. The FDA regulates Cisapride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Cisapride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Cisapride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Cisapride supplier is an individual or a company that provides Cisapride active pharmaceutical ingredient (API) or Cisapride finished formulations upon request. The Cisapride suppliers may include Cisapride API manufacturers, exporters, distributors and traders.
click here to find a list of Cisapride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Cisapride DMF (Drug Master File) is a document detailing the whole manufacturing process of Cisapride active pharmaceutical ingredient (API) in detail. Different forms of Cisapride DMFs exist exist since differing nations have different regulations, such as Cisapride USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Cisapride DMF submitted to regulatory agencies in the US is known as a USDMF. Cisapride USDMF includes data on Cisapride's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Cisapride USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Cisapride suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Cisapride as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Cisapride API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Cisapride as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Cisapride and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Cisapride NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Cisapride suppliers with NDC on PharmaCompass.
Cisapride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Cisapride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Cisapride GMP manufacturer or Cisapride GMP API supplier for your needs.
A Cisapride CoA (Certificate of Analysis) is a formal document that attests to Cisapride's compliance with Cisapride specifications and serves as a tool for batch-level quality control.
Cisapride CoA mostly includes findings from lab analyses of a specific batch. For each Cisapride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Cisapride may be tested according to a variety of international standards, such as European Pharmacopoeia (Cisapride EP), Cisapride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Cisapride USP).
