
Synopsis
0
EU WC
0
KDMF
0
VMF
0
Australia

0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
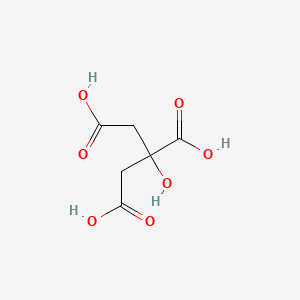

1. Anhydrous Citric Acid
2. Citrate
3. Citric Acid Monohydrate
4. Citric Acid, Anhydrous
5. Uralyt U
1. 77-92-9
2. 2-hydroxypropane-1,2,3-tricarboxylic Acid
3. Citric Acid, Anhydrous
4. Aciletten
5. Anhydrous Citric Acid
6. Citro
7. Citretten
8. Chemfill
9. Hydrocerol A
10. 1,2,3-propanetricarboxylic Acid, 2-hydroxy-
11. Citric Acid Anhydrous
12. Kyselina Citronova
13. 2-hydroxy-1,2,3-propanetricarboxylic Acid
14. 2-hydroxytricarballylic Acid
15. Caswell No. 221c
16. 3-carboxy-3-hydroxypentane-1,5-dioic Acid
17. Fema No. 2306
18. F 0001 (polycarboxylic Acid)
19. 2-hydroxypropanetricarboxylic Acid
20. Fema Number 2306
21. K-lyte
22. Kyselina Citronova [czech]
23. K-lyte Ds
24. Citric Acid,anhydrous
25. Ccris 3292
26. Hsdb 911
27. Epa Pesticide Chemical Code 021801
28. Citricum Acidum
29. Citric Acid Monoglyceride
30. Uro-trainer
31. Ai3-06286
32. Suby G
33. Nsc 30279
34. Nsc 626579
35. Brn 0782061
36. Mfcd00011669
37. Nsc-30279
38. Nsc-626579
39. Chembl1261
40. Xf417d3psl
41. Kyselina 2-hydroxy-1,2,3-propantrikarbonova [czech]
42. Kyselina 2-hydroxy-1,2,3-propantrikarbonova
43. Chebi:30769
44. .beta.-hydroxytricarballylic Acid
45. Citr
46. Nsc30279
47. Nsc626579
48. Nsc-112226
49. Citric Acid Bp
50. Citric Acid, 99%
51. Ncgc00090954-03
52. E330
53. 2-hydroxy-1,2,3-propanetricarboxyic Acid
54. Citric Acid,hydrous
55. Dsstox_cid_332
56. E 330
57. Beta-hydroxytricarballylic Acid
58. Citric Acid, Hydrous
59. Citrate Anion
60. Dsstox_rid_75520
61. Dsstox_gsid_20332
62. 141633-96-7
63. 1,2,3-propanetricarboxylic Acid, 2-hydroxy-, Homopolymer
64. Ins No.330
65. Citric Acid [usan:jan]
66. Cas-77-92-9
67. Ins-330
68. 1,3-propanetricarboxylic Acid, 2-hydroxy-
69. 10402-15-0
70. Einecs 201-069-1
71. Unii-xf417d3psl
72. E-330
73. Citraclean
74. Citronensaeure
75. Acidum Citricum
76. Citric-acid
77. Anhydrous Citrate
78. 2fwp
79. 4aci
80. 4nrm
81. H3cit
82. Citric Acid, Anhydrous [usp:jan]
83. Citric Acid,(s)
84. Citric Acid (8ci)
85. K-lyte (salt/mix)
86. 1i2s
87. 1o4l
88. 1rq2
89. 1y4a
90. 2bo4
91. 2c4v
92. 2fw6
93. 4to8
94. Citraclean (salt/mix)
95. Poly(oxy-1,2-ethanediyl), .alpha.-phosphono-.omega.-hydroxy-, C14-18-alkyl Ethers
96. Citric Acid-[13c6]
97. Citric Acid (anhydrous)
98. Spectrum3_001850
99. Wln: Qv1xqvq1vq
100. Beta-hydroxytricarballylate
101. Cid_311
102. K-lyte/cl (salt/mix)
103. Citric Acid [mi]
104. K-lyte Ds (salt/mix)
105. Acidum Citricum Monohydrate
106. Bmse000076
107. Hoc(ch2cooh)2cooh
108. Ec 201-069-1
109. Citric Acid [fhfi]
110. Citric Acid [hsdb]
111. Ncistruc1_000057
112. Ncistruc2_000099
113. Nciopen2_004062
114. Nciopen2_004502
115. Oprea1_502996
116. Bspbio_003240
117. Citric Acid Anhydrous (jan)
118. 4-03-00-01272 (beilstein Handbook Reference)
119. Citric Acid, Anhydrous, Usp
120. Mls001066346
121. Citric Acid [who-dd]
122. Citric Acid (fragrance Grade)
123. Citric Acid, Anhydrous (usp)
124. Citricum Acidum [hpus]
125. Anhydrous Citric Acid (jp17)
126. Gtpl2478
127. Citric Acid (industrial Grade)
128. Citric Acid, Analytical Standard
129. Dtxsid3020332
130. Bdbm14672
131. Citric Acid, P.a., 99.5%
132. Kbio3_002740
133. 4o61
134. Citric Acid 5% Solution In Water
135. Citric Acid, Electrophoresis Grade
136. Hms1787n01
137. Hms2268b04
138. Pharmakon1600-01300013
139. Zinc895081
140. Anhydrous Citric Acid [ii]
141. Anhydrous Citric Acid [jan]
142. Citric Acid 10% Solution In Water
143. Hy-n1428
144. Str12052
145. Tox21_113436
146. Tox21_202405
147. Tox21_300124
148. Bbl002530
149. Nsc759606
150. S5761
151. Stk286098
152. Citric Acid,anhydrous [vandf]
153. Akos000119911
154. Anhydrous Citric Acid [mart.]
155. Citric Acid, Lr, Anhydrous, >=99%
156. 2-hydroxy-1,2,3-propanetricarboxylate
157. Acidum Citricum [who-ip Latin]
158. Cs-6965
159. Db04272
160. 3-carboxy-3-hydroxypentane-1,5-dioate
161. Citric Acid, >=99.5%, Fcc, Fg
162. Citric Acid, Acs Reagent, >=99.5%
163. Citric Acid, Anhydrous Powder, A.c.s.
164. 2-hydroxy-1,3-propanetricarboxylic Acid
165. Citric Acid, Anhydrous [who-ip]
166. Ncgc00090954-01
167. Ncgc00090954-02
168. Ncgc00090954-04
169. Ncgc00090954-05
170. Ncgc00254055-01
171. Ncgc00259954-01
172. 2-hydroxypropane-1,2,3-tricarboxylicacid
173. Bp-31028
174. Citric Acid, Anhydrous Granular, A.c.s.
175. Nci60_022579
176. Smr000471840
177. Citric Acid 50% Solution In Water (w/w)
178. Sbi-0206765.p001
179. Citric Acid, Saj First Grade, >=99.5%
180. 2-hydroxy-1,2,3-propane Tricarboxylic Acid
181. 2-hydroxy-1,2,3-propanenetricarboxylic Acid
182. Anhydrous Citric Acid [usp Monograph]
183. B7297
184. C1949
185. Citric Acid, Anhydrous [ep Impurity]
186. Citric Acid, Aqueous Solution (food Grade)
187. Citric Acid, Vetec(tm) Reagent Grade, 99%
188. Ft-0623957
189. Ft-0665073
190. Ft-0728530
191. Citric Acid, Anhydrous [usp Impurity]
192. Clenpiq Component Anhydrous Citric Acid
193. C00158
194. D00037
195. Ae-562/40806920
196. Anhydrous Citric Acid Component Of Clenpiq
197. Citric Acid, Bioultra, Anhydrous, >=99.5% (t)
198. Q159683
199. J-520099
200. 1,2,3-propanetricarboxylic Acid, 2-hydroxy- (9ci)
201. Z56754862
202. Citric Acid (monohydrate): H2o = 1 G : 1 Ml Solution
203. Citric Acid, Certified Reference Material, Tracecert(r)
204. Citric Acid, Meets Usp Testing Specifications, Anhydrous
205. F2191-0222
206. 8f5d336a-442d-434a-9fb0-e400ff74e343
207. Citrate Standard For Ic, 1000 Mg/l, Analytical Standard
208. 1,2,3-propanetricarboxylic Acid,2-hydroxy (citric Acid)
209. Citric Acid, United States Pharmacopeia (usp) Reference Standard
210. Citric Acid (constituent Of Cranberry Liquid Preparation) [dsc]
211. Citric Acid, Anhydrous, Cell Culture Tested, Plant Cell Culture Tested
212. Citric Acid, Anhydrous, European Pharmacopoeia (ep) Reference Standard
213. Citric Acid, Anhydrous, Free-flowing, Redi-dri(tm), Acs Reagent, >=99.5%
214. Citric Acid (constituent Of Garcinia Cambogia And Garcinia Indica) [dsc]
215. Citric Acid, Anhydrous, Pharmaceutical Secondary Standard; Certified Reference Material
216. Citric Acid, Meets Analytical Specification Of Ph. Eur., Bp, Usp, E330, Anhydrous, 99.5-100.5% (based On Anhydrous Substance)
| Molecular Weight | 192.12 g/mol |
|---|---|
| Molecular Formula | C6H8O7 |
| XLogP3 | -1.7 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 192.02700259 g/mol |
| Monoisotopic Mass | 192.02700259 g/mol |
| Topological Polar Surface Area | 132 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 227 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Mesh Heading: Anticoagulants, chelating Agents
National Library of Medicine, SIS; ChemIDplus Record for Citric acid (77-92-9). Available from, as of April 17, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
/EXPL THER/ Regional citrate anticoagulation (RCA) is an effective form of anticoagulation for continuous renal replacement therapy (CRRT) in patients with contraindications to heparin. Its use has been very limited, possibly because of the need for special infusion solutions and difficult monitoring of the metabolic effects./The objective of this study was/ to investigate the safety and the feasibility of an RCA method for continuous veno-venous hemofiltration (CVVH) using commercially available replacement fluid. We evaluated 11 patients at high risk of bleeding, requiring CVVH. RCA was performed using commercially available replacement fluid solutions to maintain adequate acid-base balance. We adjusted the rate of citrate infusion to achieve a post-filter ionized calcium concentration [iCa] <0.4 mmol/L when blood flow was <250 mL/min, or <0.6 mmol/L when blood flow was >250 mL/min. When needed, we infused calcium gluconate to maintain systemic plasma [iCa] within the normal range. Twenty-nine filters ran for a total of 965.5 hr. Average filter life was 33.6+/-20.5 hr. Asymptomatic hypocalcemia was detected in 6.9% of all samples. No [iCa] values <0.9 mmol/L were observed. Hypercalcemia (1.39+/-0.05 mmol/L) occurred in 2.5% of all samples. /The authors/ observed hypernatremia (threshold 153 mmol/L) and alkalosis (threshold 7.51) in only 9.3% and 9.4% respectively of all samples, mostly concomitantly. No patient showed any signs of citrate toxicity. /They/ developed a protocol for RCA during CVVH using commercially available replacement fluid that proved safe, flexible and applicable in an Intensive Care Unit (ICU) setting.
PMID:17417764 Cubattoli L et al; Int J Artif Organs. 30 (3): 244-52 (2007)
It has ... been used to dissolve urinary bladder calculi, & as mild astringent.
Troy, D.B. (Ed); Remmington The Science and Practice of Pharmacy. 21 st Edition. Lippincott Williams & Williams, Philadelphia, PA 2005, p. 1085
Citrate ... of ... value in alleviation of chronic metabolic acidosis ... from chronic renal insufficiency or syndrome of renal tubular acidosis ... usually prescribed in form of sodium citrate and citric acid soln, USP ...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 806
Potassium citrate, up to 10 g daily, has been used as a potassium supplement; the potassium and sodium salts have been used, in similar dosages, as mild diuretics in humans.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 5:768
A study of abdominal pain and severity of other side effects attributed to Picolax, a combination of citric acid, magnesium oxide and sodium picosulfate, was conducted among 267 patients, 55 of whom had inflammatory bowel disease, all of whom were given a full single dose of Picolax as preparation for radiology or endoscopy. The frequency of increased abdominal pain and severe side effects after Picolax administration was similar in the patients with inflammatory bowel disease and the patients with other colonic disorders. None of the patients with iron deficiency in whom investigations had yielded negative results reported severe side effects; this was significantly different from the proportion reporting severe side effects among the patients with inflammatory bowel disease, the irritable bowel syndrome and diverticular disease. The increase in the mean number of stools/24 hr after Picolax was lower in the patients with inflammatory bowel disease than in the other diagnostic groups. On review 2-4 wk after examination none of the patients with inflammatory bowel disease reported deterioration in their symptoms.
McDonagh AJ et al; Br Med J 299: 776-7 (1989)
Following the occurrence of aluminum encephalopathy in four patients with chronic renal failure, 34 azotemic patients seen during the same year and five volunteers who took varying combinations of aluminum hydroxide and an alkalinizing citrate (Shohl's) solution were studied. It was found that the four encephalopathic cases were older than the 34 azotemic patients (68 years + or - 14 standard deviation, versus 50 + or - 13, p< 0.05), had a higher mean serum aluminum value (727 ug/l + or - 320 versus 92 + or - 73, p< 0.005), had taken more aluminum hydroxide (5 g/day + or - 0.9 versus 1.6 + or - 1.8, p< 0.01), and more Shohl's solution (64 ml/day + or - 19 versus 20 + or - 29, p< 0.01). In all 38 patients the serum aluminum values correlated directly with age (p=0.01), aluminum hydroxide (p=0.001) and concomitant citrate intake (p=0.004). In the five healthy volunteers the 24 hr urinary aluminum excretion increased from a baseline of 22 ug + or - 19 standard deviation to 167 + or - 109 (p=0.05) during aluminum hydroxide intake, rising to 580 + or - 267 (p=0.01) during the simultaneous intake of citrate and aluminum hydroxide. Corresponding serum aluminum values were 11 ug/l + or - 2 standard deviation, 44 + or - 34 (p= 0.1), and 98 + or - 58 (p<0.05). Thus citrate seems to enhance aluminum absorption and may cause encephalopathy in patients with chronic renal failure, especially the elderly.
PMID:2914409 Bakir AA et al; Clin Nephrol 31 (1): 40-4 (1989)
Anticoagulants
Agents that prevent BLOOD CLOTTING. (See all compounds classified as Anticoagulants.)
Calcium Chelating Agents
Substances that bind to and sequester CALCIUM ions. (See all compounds classified as Calcium Chelating Agents.)
A - Alimentary tract and metabolism
A09 - Digestives, incl. enzymes
A09A - Digestives, incl. enzymes
A09AB - Acid preparations
A09AB04 - Citric acid
/A portion/ of the circulating (mainly metabolic but also ingested) citric acid is excreted in urine, with 24-hour urine reference values between 1.5 and 3.68 mmol, corresponding to 0.29-0.71 g citric acid excreted per person per day.
United Nations Environment Programme: OECD; Screening Information Data Sheets on Citric Acid (77-92-9) (January 1997). Available from, as of May 9, 2006: https://www.chem.unep.ch/irptc/sids/OECDSIDS/sidspub.html
Citric acid is a normal metabolite and an intermediate in cellular oxidative metabolism ... The acid is formed in the mitochondrion after condensation of acetate with oxaloacetate. The six-carbon acid is then successively degraded to a series of four-carbon acids, effectively accomplishing oxidation of acetate in the cell.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 5:767
In human (as well as in animal and plant) physiology, citric acid is a very common intermediate in one of the central biochemical cycles, the Krebs or tricarboxylic acid cycle, which takes place in every cell. It completes the breakdown of pyruvate formed from glucose through glycolysis, thereby liberating carbon dioxide and a further four hydrogen atoms which are picked up by electron transport molecules. Thus, in man approximately 2 kg of citric acid are formed and metabolised every day. This physiological pathway is very well developed and capable of processing very high amounts of citric acid as long as it occurs in low concentrations.
United Nations Environment Programme: OECD; Screening Information Data Sheets on Citric Acid (77-92-9) (January 2001). Available from, as of May 9, 2006: https://www.chem.unep.ch/irptc/sids/OECDSIDS/sidspub.html
Citric acid in reaction with enzyme citratase /citrate lyase/ yields oxaloacetic acid & acetic acid.
Fenaroli's Handbook of Flavor Ingredients. Volume 2. Edited, translated, and revised by T.E. Furia and N. Bellanca. 2nd ed. Cleveland: The Chemical Rubber Co., 1975., p. 785
... The NK(2), and to a lesser extent the NK(1), receptors have been shown to be involved with citric acid-induced bronchoconstriction in the guinea pig, which is in part mediated by endogenously released bradykinin. Tachykinins and bradykinin could also modulate citric acid-induced bronchoconstriction. ... Bronchoconstriction induced by citric acid inhalation in the guinea pig, mainly caused by the tachykinin NK(2) receptor, is counteracted by bronchoprotective NO after activation of bradykinin B(2) and tachykinin NK(1) receptors in airway epithelium.
PMID:11749919 Ricciardolo FL; Am J Med 111 (Suppl 8A): 18S-24S (2001)
... A concentration of 47.6 mmol/L of citric acid (pH 2.3) in water led to total cell death within three minutes of incubation /with gingival fibroblasts (GF)/. Media containing 23.8 mmol/L and 47.6 mmol/L of citric acid exerted strong cytotoxicity (47 to 90 per cent of cell death) and inhibited protein synthesis (IC50 = 0.28 per cent) of GF within three hours of incubation. Incubation of cells in a medium containing 11.9 mmol/L of citric acid also suppressed the attachment and spreading of fibroblasts on culture plates and Type I collagen, with 58 per cent and 22 per cent of inhibition, respectively. Culture medium supplemented with 11.9, 23.8 and 47.6 mmol/L of citric acid also led to extracellular acidosis by decreasing the pH value from 7.5 to 6.3, 5.2 and 3.8, respectively.
PMID:10452169 Lan WC et al; Aust Dent J 44 (2): 123-30 (1999)
Inhalation of citric acid (CA) causes airway constriction and coughing. To investigate the role of mast cells in CA-induced airway constriction and cough, three experiments using guinea pigs were carried out. In the first experiment, /the authors/ used compound 48/80 to deplete mast cells, cromolyn sodium to stabilize mast cells, MK-886 to inhibit synthesis of leukotrienes, pyrilamine to antagonize histamine H1 receptor, methysergide to antagonize serotonin receptor, and indomethacin to inhibit cyclooxygenase. In the second experiment, compound 48/80-pretreated animals were divided into 2 parts; the first one was used to test the role of exogenous leukotriene (LT) C4, while the second one to test the role of exogenous histamine. Decreases in respiratory compliance (Crs) and forced expiratory volume in 0.1 sec (FEV0.1) were used as indicators for airway constriction in anesthetized guinea pigs. CA-induced cough was recorded for 12 min using a barometric body plethysmograph in conscious animals. In the third experiment, the activation of mast cells upon CA inhalation was investigated by determining lung tissue or arterial plasma histamine concentration in animals. Exposure to CA induced marked airway constriction and increase in cough number. Compound 48/80, cromolyn sodium, MK-886 and pyrilamine, but not indomethacin or methysergide, significantly attenuated CA-induced airway constriction and cough. Injection of LTC4 or histamine caused a significant increase in CA-induced airway constriction and cough in compound 48/80-pretreated animals. In addition, CA inhalation caused significant increase in lung tissue and plasma histamine concentrations, which were blocked by compound 48/80 pretreatment. These results suggest that mast cells play an important role in CA aerosol inhalation-induced airway constriction and cough via perhaps mediators including LTs and histamine.
PMID:20359123 Lai YL et al; Chin J Physiol. 52 (5 Suppl): 332-8 (2009)
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2013-10-07
Pay. Date : 2013-09-27
DMF Number : 23078
Submission : 2009-08-28
Status : Active
Type : II

Certificate Number : CEP 2016-005 - Rev 01
Issue Date : 2024-08-28
Type : Chemical
Substance Number : 455
Status : Valid


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 25371
Submission : 2011-10-07
Status : Active
Type : II




Registration Number : 218MF10737
Registrant's Address : 1451 Kaseda Kawabata, Minamisatsuma City, Kagoshima Prefecture
Initial Date of Registration : 2006-09-06
Latest Date of Registration : --




Registration Number : 224MF10212
Registrant's Address : Matsuura Sakaisuji Honmachi Building, 1-9-28, Kyutaro-cho, Chuo-ku, Osaka City, Osaka Prefecture
Initial Date of Registration : 2012-10-29
Latest Date of Registration : --



 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?