
Synopsis
Synopsis
0
VMF
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
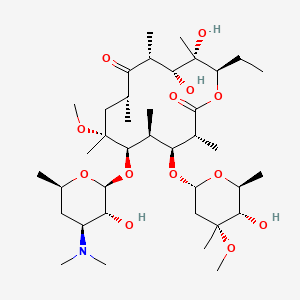

1. 6-o-methylerythromycin
2. A 56268
3. A-56268
4. A56268
5. Biaxin
6. Te 031
7. Te-031
8. Te031
1. 81103-11-9
2. Biaxin
3. 6-o-methylerythromycin
4. Klaricid
5. 6-o-methylerythromycin A
6. Abbott-56268
7. Clarithromycine
8. Clathromycin
9. Macladin
10. Klacid
11. Veclam
12. Erythromycin, 6-o-methyl-
13. Mavid
14. Naxy
15. A-56268
16. Te-031
17. Clarith
18. Cyllind
19. Kofron
20. Zeclar
21. Clarithromycinum
22. Clarithromycin Identity
23. Biaxin Xl
24. Te031
25. Nsc-758704
26. Prevpac
27. Chebi:3732
28. H1250jik0a
29. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-(((2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-14-ethyl-12,13-dihydroxy-4-(((2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl)oxy)-7-methoxy-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione
30. Claritromicina
31. Abbotic
32. Astromen
33. Bicrolid
34. Clacine
35. Clambiotic
36. Claribid
37. Claricide
38. Claridar
39. Claripen
40. Fromilid
41. Heliclar
42. Klaciped
43. Mabicrol
44. Clacee
45. Clacid
46. Clarem
47. Crixan
48. Cyllid
49. Klabax
50. Klarid
51. Klarin
52. Maclar
53. Helas
54. Adel
55. Biaxin Filmtab
56. Klax
57. Biaxin Hp
58. Biaxin Xl Filmtab
59. Klaricid Pediatric
60. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-12,13-dihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-7-methoxy-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione
61. Cla
62. Clarithromycin Extended Release
63. Drg-0099
64. Klaricid H.p.
65. Cty
66. Smr000466382
67. Clarithromycine [inn-french]
68. Clarithromycinum [inn-latin]
69. Claritromicina [inn-spanish]
70. Sr-05000001992
71. Clarithromycin (biaxin, Klacid)
72. Clarithromycina
73. Unii-h1250jik0a
74. Clarosip
75. Ccris 8833
76. Klaricid Xl
77. Hsdb 8055
78. (14r)-14-hydroxyclarithromycin
79. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-14-ethyl-12,13-dihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyl-tetrahydropyran-2-yl]oxy-7-methoxy-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione
80. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-12,13-dihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-7-methoxy
81. Clarithromycin,(s)
82. Biaxin (tn)
83. Spectrum_000089
84. Cpd000466382
85. Clarithromycin [usan:usp:inn:ban:jan]
86. Specplus_000559
87. 6-o-methyl Erythromycin
88. O(6)-methylerythromycin
89. Spectrum2_001668
90. Spectrum3_001667
91. Spectrum4_000629
92. Spectrum5_001729
93. 6-0-methylerythromycin A
94. A56268
95. Clarithromycin [mi]
96. Chembl1741
97. Clarithromycin [inn]
98. Clarithromycin [jan]
99. Schembl38125
100. Bspbio_003453
101. Kbiogr_001218
102. Kbioss_000509
103. Mls000759516
104. Mls001201751
105. Mls001424066
106. Bidd:gt0200
107. Clarithromycin [vandf]
108. Divk1c_006655
109. Spectrum1504231
110. Spbio_001855
111. Clarithromycin [mart.]
112. Lactoferrin B & Clarithromycin
113. Lactoferrin H & Clarithromycin
114. Clarithromycin [usp-rs]
115. Clarithromycin [who-dd]
116. Dtxsid3022829
117. Clarithromycin & Interleukin-12
118. Clm & Il-12
119. Gtpl10903
120. Kbio1_001599
121. Kbio2_000509
122. Kbio2_003077
123. Kbio2_005645
124. Kbio3_002673
125. Anx-015
126. Sdp-015
127. Clarithromycin (jp17/usp/inn)
128. Clarithromycin, >=95% (hplc)
129. Clarithromycin, >=98% (hplc)
130. Hms1922h09
131. Hms2051g18
132. Hms2090o11
133. Hms2094m05
134. Hms2231a08
135. Hms3715j17
136. Pharmakon1600-01504231
137. Clarithromycin [orange Book]
138. Bdbm50404044
139. Ccg-39086
140. Clarithromycin [ep Monograph]
141. Clarithromycin [usp Impurity]
142. Lmpk04000014
143. Nsc758704
144. S2555
145. Zinc85534098
146. Clarithromycin [usp Monograph]
147. Akos015894242
148. Prevpac Component Clarithromycin
149. Cs-2576
150. Db01211
151. Nc00140
152. Nsc 758704
153. Clarithromycin Identity [usp-rs]
154. Ncgc00178054-01
155. Ncgc00178054-06
156. Clarithromycin 100 Microg/ml In Methanol
157. Clarithromycin Component Of Prevpac
158. Hy-17508
159. Sbi-0206716.p001
160. Clarithromycin 100 Microg/ml In Acetonitrile
161. C06912
162. D00276
163. F14975
164. Ab00053394-10
165. Ab00053394-12
166. Ab00053394-13
167. Ab00053394_14
168. Ab00053394_15
169. 103c119
170. A840042
171. Q118551
172. Q-200870
173. Sr-05000001992-1
174. Sr-05000001992-2
175. Brd-k49668410-001-07-1
176. Brd-k49668410-001-18-8
177. Clarithromycin, European Pharmacopoeia (ep) Reference Standard
178. Clarithromycin, United States Pharmacopeia (usp) Reference Standard
179. Clarithromycin Identity, United States Pharmacopeia (usp) Reference Standard
180. Clarithromycin, Ready Made Solution, 50 Mg/ml In Dmso, 0.2 Mum Filtered
181. Clarithromycin For Peak Identification, European Pharmacopoeia (ep) Reference Standard
182. Clarithromycin, Pharmaceutical Secondary Standard; Certified Reference Material
183. (3r,4s,5r,6r,7r,9r,11r,12r,13s,14r)-6-[(2s,3r,4r,6r)-4-(dimethylamino)-3-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-14-ethyl-12,13-dihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyl-tetrahydropyran-2-yl]oxy-7-methoxy-3,5,7,9,11,13-hexamethyl-oxacy
184. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-((2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yloxy)-14-ethyl-12,13-dihydroxy-4-((2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yloxy)-7-methoxy-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione
185. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-12,13-dihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-7-methoxy-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dion
186. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-{[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl]oxy}-14-ethyl-12,13-dihydroxy-4-{[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl]oxy}-7-methoxy-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione (non-preferred Name)
187. (3r,4s,5s,6r,7r,9r,11s, 12r,13s,14s)-6-{[(2s,3r,4s,6r)- 4-dimethylamino-3-hydroxy-6-methyloxan-2-yl]oxy}-14-ethyl-12,13-dihydroxy- 4-{[(2r,4s,5s,6s)-5-hydroxy-4-methoxy-4,6- Dimethyloxan-2-yl]oxy}-7-methoxy-3,5,7,9,11,13-hexamethyl-1- Oxacyclotetradecane-2,10-dione
| Molecular Weight | 748.0 g/mol |
|---|---|
| Molecular Formula | C38H69NO13 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 8 |
| Exact Mass | 747.47689126 g/mol |
| Monoisotopic Mass | 747.47689126 g/mol |
| Topological Polar Surface Area | 183 Ų |
| Heavy Atom Count | 52 |
| Formal Charge | 0 |
| Complexity | 1190 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 18 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Biaxin |
| PubMed Health | Clarithromycin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Clarithromycin is a semi-synthetic macrolide antibiotic. Chemically, it is 6-0-methylerythromycin. The molecular formula is C38H69NO13, and the molecular weight is 747.96. The structural formula is: Clarithromycin is a white to off-white crystalline... |
| Active Ingredient | Clarithromycin |
| Dosage Form | Treatment; Tablet; For suspension |
| Route | mac; Oral |
| Strength | 250mg; 125mg/5ml; 500mg; 250mg/5ml |
| Market Status | Prescription |
| Company | Abbott; Abbvie |
| 2 of 6 | |
|---|---|
| Drug Name | Biaxin xl |
| PubMed Health | Clarithromycin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Clarithromycin is a semi-synthetic macrolide antibiotic. Chemically, it is 6-0-methylerythromycin. The molecular formula is C38H69NO13, and the molecular weight is 747.96. The structural formula is:Clarithromycin is a white to off-white crystalline p... |
| Active Ingredient | Clarithromycin |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Abbvie |
| 3 of 6 | |
|---|---|
| Drug Name | Clarithromycin |
| Active Ingredient | Clarithromycin |
| Dosage Form | Tablet, extended release; Tablet; For suspension |
| Route | Oral |
| Strength | 250mg; 125mg/5ml; 500mg; 250mg/5ml |
| Market Status | Prescription |
| Company | Wockhardt; Ranbaxy; Teva; Actavis Labs Fl; Apotex; Aurobindo; Allied Pharma; Sandoz; Mylan; Roxane |
| 4 of 6 | |
|---|---|
| Drug Name | Biaxin |
| PubMed Health | Clarithromycin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Clarithromycin is a semi-synthetic macrolide antibiotic. Chemically, it is 6-0-methylerythromycin. The molecular formula is C38H69NO13, and the molecular weight is 747.96. The structural formula is: Clarithromycin is a white to off-white crystalline... |
| Active Ingredient | Clarithromycin |
| Dosage Form | Treatment; Tablet; For suspension |
| Route | mac; Oral |
| Strength | 250mg; 125mg/5ml; 500mg; 250mg/5ml |
| Market Status | Prescription |
| Company | Abbott; Abbvie |
| 5 of 6 | |
|---|---|
| Drug Name | Biaxin xl |
| PubMed Health | Clarithromycin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Clarithromycin is a semi-synthetic macrolide antibiotic. Chemically, it is 6-0-methylerythromycin. The molecular formula is C38H69NO13, and the molecular weight is 747.96. The structural formula is:Clarithromycin is a white to off-white crystalline p... |
| Active Ingredient | Clarithromycin |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Abbvie |
| 6 of 6 | |
|---|---|
| Drug Name | Clarithromycin |
| Active Ingredient | Clarithromycin |
| Dosage Form | Tablet, extended release; Tablet; For suspension |
| Route | Oral |
| Strength | 250mg; 125mg/5ml; 500mg; 250mg/5ml |
| Market Status | Prescription |
| Company | Wockhardt; Ranbaxy; Teva; Actavis Labs Fl; Apotex; Aurobindo; Allied Pharma; Sandoz; Mylan; Roxane |
Anti-Bacterial Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2012)
Oral clarithromycin is used in combination with amoxicillin and lansoprazole or omeprazole (triple therapy) for the treatment of Helicobacter pylori infection and duodenal ulcer disease. Clarithromycin also is used orally in combination with omeprazole (dual therapy) or ranitidine bismuth citrate for the treatment of H. pylori infection in patients with an active duodenal ulcer. Clarithromycin also has been used orally in other multiple-drug regimens (with or without amoxicillin, lansoproprazole, omeprazole, or ranitidine bismuth citrate) for the treatment of H. pylori infection associated with peptic ulcer disease. /Included in US product labeling/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 242
Clarithromycin is used orally for the treatment of pharyngitis and tonsillitis, mild to moderate respiratory tract infections (acute bacterial exacerbation of chronic bronchitis, acute maxillary sinusitis, community-acquired pneumonia), uncomplicated skin and skin structure infections, and acute otitis media caused by susceptible organisms. Clarithromycin also is used orally in the treatment of disseminated infections caused by Mycobacterium avium complex (MAC) in patients with advanced human immunodeficiency virus (HIV) infection and for prevention of disseminated MAC infection (both primary and secondary prophylaxis) in HIV-infected individuals. /Included in US product labeling/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 242
Clarithromycin (conventional tablets) is used in conjunction with amoxicillin and lansoprazole or omeprazole (triple therapy) for the treatment of Helicobacter pylori (formerly Campylobacter pylori or C. pyloridis) infection in patients with duodenal ulcer disease (active or up to 1-year history of duodenal ulcer). Clarithromycin also is used in conjunction with omeprazole (dual therapy) or ranitidine bismuth citrate for the treatment of H. pylori infection in patients with an active duodenal ulcer. /Included in US product labeling/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 243
For more Therapeutic Uses (Complete) data for Clarithromycin (17 total), please visit the HSDB record page.
A total of 38 patients with clarithromycin-induced neurotoxicity have been reported. The average age of patients was 51.3 years (range: 19-87 years) with females comprising 52.6% of patients. Psychiatric illness was the most common comorbidity, while only two patients had renal failure. Clarithromycin had been prescribed for respiratory infections in most patients, and only two patients were receiving more than 1000 mg/day of antibiotic. The symptoms started 1 day to 10 days after starting clarithromycin (mean: 5 days). A total of 71% of patients were under treatment with concomitant medication, and eight patients were undergoing treatment with psychoactive drugs. Patients had a very good outcome after clarithromycin was discontinued, but medication with neuroleptics or benzodiazepine was required for 58% of patients in the acute phase. Only four patients underwent an electroencephalogram (EEG). Our illustrative patient was a 74-year-old woman with clarithromycin-induced delirium due to non-convulsive status epilepticus (NCSE). Her clinical symptoms and electroencephalogram (EEG) readings dramatically improved after discontinuation of clarithromycin. The mechanism underlying the central nervous system side effects remains unclear. We suggest including an EEG in the diagnostic procedures of patients under treatment with clarithromycin who develop features of neurotoxicity because an EEG can help to differentiate patients with psychiatric illness from those with encephalopathy or epilepsy. Because of the widespread use of clarithromycin, clinicians should be aware of its neurotoxicity. Early detection of clarithromycin-induced neurotoxicity and discontinuation of the drug may result in full recovery.
PMID:21269833 Bandettini di Poggio M et al; J Clin Neurosci 18 (3): 313-8 (2011)
/The investigators/ treated 13 elderly patients with chronic mycobacterial lung disease with clarithromycin using 1000 mg b.i.d. as monotherapy. Patients had a mean age of 70 years, and 12 of 13 had creatinine clearances of 31-71 ml/min. Adverse events were seen in 100% of patients, with the most common being bitter taste (92%), nausea (92%), vomiting (54%) and central nervous system symptoms (54%). Elevated liver enzymes developed in five (38%) of 13 patients at weeks 1-6 of therapy. Mean serum levels of clarithromycin plus its 14-OH metabolite were 12.9 +/- 3.6 micrograms/ml (SD). There were 11 patients (85%) who discontinued the high dose within 3 months because of side effects. Serum drug levels of clarithromycin plus its 14-OH metabolite consistently exceeded 12 micrograms/ml in six of six patients who discontinued drug (10 of 10 values) compared with neither of two patients who tolerated the high dose (0 of 6 values). A dose reduction to 500 mg b.i.d. was well tolerated (nine of 10 patients). Future trials with clarithromycin in this population should use lower doses with attention to body mass and renal function to minimize side effects.
PMID:8477575 Wallace RJ Jr et al; Diagn Microbiol Infect Dis 16 (3): 215-21 (1993)
Corneal opacities have occurred in animals at clarithromycin dosages 8- 12 times the maximum recommended human dosage (on a mg/m2 basis).
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 249
Other adverse effects reported with combined clarithromycin-omeprazole therapy that differed from those reported with omeprazole alone included rhinitis (2% of patients), pharyngitis (1% of patients), and flu syndrome (1% of patients).
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 249
For more Drug Warnings (Complete) data for Clarithromycin (22 total), please visit the HSDB record page.
An alternative medication for the treatment of acute otitis media caused by H. influenzae, M. catarrhalis, or S. pneumoniae in patients with a history of type I penicillin hypersensitivity. Also for the treatment of pharyngitis and tonsillitis caused by susceptible Streptococcus pyogenes, as well as respiratory tract infections including acute maxillary sinusitis, acute bacterial exacerbations of chronic bronchitis, mild to moderate community-acquired pneuomia, Legionnaires' disease, and pertussis. Other indications include treatment of uncomplicated skin or skin structure infections, helicobacter pylori infection, duodenal ulcer disease, bartonella infections, early Lyme disease, and encephalitis caused by Toxoplasma gondii (in HIV infected patients in conjunction with pyrimethamine). Clarithromycin may also decrease the incidence of cryptosporidiosis, prevent the occurence of -hemolytic (viridans group) streptococcal endocarditis, as well as serve as a primary prevention for Mycobacterium avium complex (MAC) bacteremia or disseminated infections (in adults, adolescents, and children with advanced HIV infection).
FDA Label
Treatment of Helicobacter spp. infections
Treatment of Helicobacter spp. infections
Clarithromycin is a macrolide antibiotic whose spectrum of activity includes many gram-positive (Staphylococcus aureus, S. pneumoniae, and S. pyogenes) and gram-negative aerobic bacteria (Haemophilus influenzae, H. parainfluenzae, and Moraxella catarrhalis), many anaerobic bacteria, some mycobacteria, and some other organisms including Mycoplasma, Ureaplasma, Chlamydia, Toxoplasma, and Borrelia. Other aerobic bacteria that clarithromycin has activity against include C. pneumoniae and M. pneumoniae. Clarithromycin has an in-vitro activity that is similar or greater than that of erythromycin against erythromycin-susceptible organisms. Clarithromycin is usually bacteriostatic, but may be bactericidal depending on the organism and the drug concentration.
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Cytochrome P-450 CYP3A Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inhibitors.)
J01FA09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J01FA09
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01F - Macrolides, lincosamides and streptogramins
J01FA - Macrolides
J01FA09 - Clarithromycin
Absorption
Clarithromycin is well-absorbed, acid stable and may be taken with food.
Route of Elimination
After a 250 mg tablet every 12 hours, approximately 20% of the dose is excreted in the urine as clarithromycin, while after a 500 mg tablet every 12 hours, the urinary excretion of clarithromycin is somewhat greater, approximately 30%.
Limited data are available on the distribution of clarithromycin in humans. Clarithromycin and 14-hydroxyclarithromycin appear to be distributed into most body tissues and fluids. Because of high intracellular concentrations of the drug, tissue concentrations are higher than serum concentrations. High concentrations of clarithromycin were present in tissue samples obtained from patients undergoing surgery. In patients who received 250-500 mg of clarithromycin orally every 12 hours for 3 days prior to surgery, peak clarithromycin concentrations in lung, tonsils, and nasal mucosa reportedly were attained 4 hours after administration and averaged 13.5-17.5, 5.3-6.5, and 5.9-8.3 mg/ kg, respectively; however, it has been suggested that these data may represent an overestimate of clarithromycin tissue concentrations because of the microbiologic assay's inability to distinguish between parent drug and its active metabolite. In children receiving clarithromycin suspension for otitis media at a dosage of 7.5 mg/kg every 12 hours for 5 doses, peak clarithromycin and 14- hydroxyclarithromycin concentrations in middle ear fluid were 2.5 and 1.3 ug/ mL, respectively; concomitant serum concentrations were 1.7 and 0.8 ug/mL, respectively. Results of studies in animals given radiolabeled clarithromycin or erythromycin indicate higher and more prolonged activity of clarithromycin in various body tissues, particularly the lung
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 254
Clarithromycin is absorbed rapidly from the GI tract after oral administration; GI absorption of the drug exceeds that of erythromycin.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 254
Clarithromycin is eliminated by both renal and nonrenal mechanisms.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 255
Following oral administration of a single 250-mg dose of radiolabeled clarithromycin in healthy men, approximately 38% of the dose (18% as clarithromycin) was excreted in urine, and 40% in feces (4% as clarithromycin), over 5 days. With oral administration of 250 or 500 mg of clarithromycin as tablets every 12 hours, approximately 20 or 30% of the respective dose is excreted unchanged in urine within 12 hours. After an oral clarithromycin dosage of 250 mg every 12 hours as the suspension, approximately 40% of the administered dose is excreted unchanged in urine. The principal metabolite found in urine is 14-hydroxyclarithromycin, which accounts for approximately 10-15% of the dose following administration of 250 or 500 mg of clarithromycin as tablets.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 255
For more Absorption, Distribution and Excretion (Complete) data for Clarithromycin (6 total), please visit the HSDB record page.
Hepatic - predominantly metabolized by CYP3A4 resulting in numerous drug interactions.
The principal metabolite found in urine is 14-hydroxyclarithromycin, which accounts for approximately 10-15% of the dose following administration of 250 or 500 mg of clarithromycin as tablets.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 255
Clarithromycin is extensively metabolized in the liver, principally by oxidative N- demethylation and hydroxylation at the 14 position; hydrolytic cleavage of the cladinose sugar moiety also occurs in the stomach to a minor extent. Although at least 7 metabolites of clarithromycin have been identified, 14-hydroxyclarithromycin is the principal metabolite in serum and the only one with substantial antibacterial activity. While both the R- and S-epimers of 14-hydroxyclarithromycin are formed in vivo, the R-epimer is present in greater amounts and has the greatest antimicrobial activity. Metabolism of clarithromycin appears to be saturable since the amount of 14-hydroxyclarithromycin after an 800-mg dose of the parent drug is only marginally greater than that after a 250-mg dose.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 255
Following oral administration of a single 250-mg dose of radiolabeled clarithromycin in healthy men, approximately 38% of the dose (18% as clarithromycin) was excreted in urine, and 40% in feces (4% as clarithromycin), over 5 days. ... The principal metabolite found in urine is 14-hydroxyclarithromycin, which accounts for approximately 10-15% of the dose following administration of 250 or 500 mg of clarithromycin as tablets.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 255
3-4 hours
Following oral administration of single 250-mg or 1.2-g doses of clarithromycin conventional tablets in healthy men, the elimination half-life averaged 4 or 11 hours, respectively. During multiple dosing every 12 hours, the elimination half-life of clarithromycin reportedly increased from 3-4 hours following a 250-mg dose (conventional tablets) every 12 hours to 5-7 hours following a 500-mg dose every 8-12 hours; the half-life of 14-hydroxyclarithromycin increased from 5-6 hours with a 250-mg dose to 7-9 hours with a 500-mg dose. When clarithromycin is administered as the oral suspension, the elimination half-life of the drug and of its 14-hydroxy metabolite appear to be similar to those observed at steady-state following administration of equivalent doses of clarithromycin as tablets.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 254
Clarithromycin is first metabolized to 14-OH clarithromycin, which is active and works synergistically with its parent compound. Like other macrolides, it then penetrates bacteria cell wall and reversibly binds to domain V of the 23S ribosomal RNA of the 50S subunit of the bacterial ribosome, blocking translocation of aminoacyl transfer-RNA and polypeptide synthesis. Clarithromycin also inhibits the hepatic microsomal CYP3A4 isoenzyme and P-glycoprotein, an energy-dependent drug efflux pump.
Clarithromycin usually is bacteriostatic, although it may be bactericidal in high concentrations or against highly susceptible organisms. Bactericidal activity has been observed against Streptococcus pyogenes, S. pneumoniae, Haemophilus influenzae, and Chlamydia trachomatis. Clarithromycin inhibits protein synthesis in susceptible organisms by penetrating the cell wall and binding to 50S ribosomal subunits, thereby inhibiting translocation of aminoacyl transfer-RNA and inhibiting polypeptide synthesis. The site of action of clarithromycin appears to be the same as that of erythromycin, clindamycin, lincomycin, and chloramphenicol.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 255

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
48
PharmaCompass offers a list of Clarithromycin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Clarithromycin manufacturer or Clarithromycin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Clarithromycin manufacturer or Clarithromycin supplier.
PharmaCompass also assists you with knowing the Clarithromycin API Price utilized in the formulation of products. Clarithromycin API Price is not always fixed or binding as the Clarithromycin Price is obtained through a variety of data sources. The Clarithromycin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Clarithromycin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Clarithromycin, including repackagers and relabelers. The FDA regulates Clarithromycin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Clarithromycin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Clarithromycin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Clarithromycin supplier is an individual or a company that provides Clarithromycin active pharmaceutical ingredient (API) or Clarithromycin finished formulations upon request. The Clarithromycin suppliers may include Clarithromycin API manufacturers, exporters, distributors and traders.
click here to find a list of Clarithromycin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Clarithromycin DMF (Drug Master File) is a document detailing the whole manufacturing process of Clarithromycin active pharmaceutical ingredient (API) in detail. Different forms of Clarithromycin DMFs exist exist since differing nations have different regulations, such as Clarithromycin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Clarithromycin DMF submitted to regulatory agencies in the US is known as a USDMF. Clarithromycin USDMF includes data on Clarithromycin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Clarithromycin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Clarithromycin suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Clarithromycin Drug Master File in Japan (Clarithromycin JDMF) empowers Clarithromycin API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Clarithromycin JDMF during the approval evaluation for pharmaceutical products. At the time of Clarithromycin JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Clarithromycin suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Clarithromycin Drug Master File in Korea (Clarithromycin KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Clarithromycin. The MFDS reviews the Clarithromycin KDMF as part of the drug registration process and uses the information provided in the Clarithromycin KDMF to evaluate the safety and efficacy of the drug.
After submitting a Clarithromycin KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Clarithromycin API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Clarithromycin suppliers with KDMF on PharmaCompass.
A Clarithromycin CEP of the European Pharmacopoeia monograph is often referred to as a Clarithromycin Certificate of Suitability (COS). The purpose of a Clarithromycin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Clarithromycin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Clarithromycin to their clients by showing that a Clarithromycin CEP has been issued for it. The manufacturer submits a Clarithromycin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Clarithromycin CEP holder for the record. Additionally, the data presented in the Clarithromycin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Clarithromycin DMF.
A Clarithromycin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Clarithromycin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Clarithromycin suppliers with CEP (COS) on PharmaCompass.
A Clarithromycin written confirmation (Clarithromycin WC) is an official document issued by a regulatory agency to a Clarithromycin manufacturer, verifying that the manufacturing facility of a Clarithromycin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Clarithromycin APIs or Clarithromycin finished pharmaceutical products to another nation, regulatory agencies frequently require a Clarithromycin WC (written confirmation) as part of the regulatory process.
click here to find a list of Clarithromycin suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Clarithromycin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Clarithromycin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Clarithromycin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Clarithromycin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Clarithromycin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Clarithromycin suppliers with NDC on PharmaCompass.
Clarithromycin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Clarithromycin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Clarithromycin GMP manufacturer or Clarithromycin GMP API supplier for your needs.
A Clarithromycin CoA (Certificate of Analysis) is a formal document that attests to Clarithromycin's compliance with Clarithromycin specifications and serves as a tool for batch-level quality control.
Clarithromycin CoA mostly includes findings from lab analyses of a specific batch. For each Clarithromycin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Clarithromycin may be tested according to a variety of international standards, such as European Pharmacopoeia (Clarithromycin EP), Clarithromycin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Clarithromycin USP).
