
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
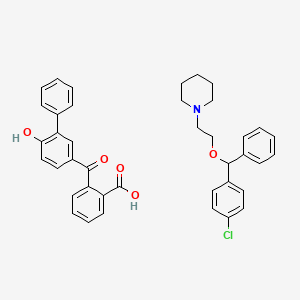

1. 85187-37-7
2. Hustazol
3. Nitossil
4. Sekin
5. Cloperastine Fendizoate [jan]
6. Einecs 286-126-9
7. Hustazol (tn)
8. Unii-2m105305su
9. 2m105305su
10. 1-[2-[(4-chlorophenyl)-phenylmethoxy]ethyl]piperidine;2-(4-hydroxy-3-phenylbenzoyl)benzoic Acid
11. Chebi:31419
12. Cloperastine Fendizoate [mart.]
13. Cloperastine Fendizoate [who-dd]
14. O-((2'-hydroxy(1,1'-biphenyl)-4-yl)carbonyl)benzoic Acid, Compoundwith 1-(2-(4-chlorobenzhydryloxy)ethyl)piperidine (1:1)
15. Cloperastine Fendizoate (jan)
16. Cloperastine Fendizoate (mart.)
17. 1-(2-((4-chlorophenyl)(phenyl)methoxy)ethyl)piperidine 2-(6-hydroxy-[1,1'-biphenyl]-3-carbonyl)benzoate
18. 1-[2-[(4-chlorophenyl)phenylmethoxy]ethyl]-hydroxy[1,1'-biphenyl]-3-yl)carbonyl]benzoate Piperidine
19. Cloperastinefendizoate
20. Cloperastine Fendizoate (jp18)
21. Dtxsid001005645
22. Hy-b2179
23. Mfcd01661515
24. Akos040740750
25. Cs-7697
26. Bs-44741
27. Da-51971
28. Sy317590
29. D01569
30. E74488
31. J-520104
32. Q27254909
33. 1-[2-[(4-chlorophenyl)(phenyl)methoxy]ethyl]piperidine 2-(6-hydroxybiphenyl-3-carbonyl)benzoate
34. 2-(6-hydroxy[1,1'-biphenyl]-3-carbonyl)benzoic Acid--1-{2-[(4-chlorophenyl)(phenyl)methoxy]ethyl}piperidine (1/1)
| Molecular Weight | 648.2 g/mol |
|---|---|
| Molecular Formula | C40H38ClNO5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 10 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 87.1 |
| Heavy Atom Count | 47 |
| Formal Charge | 0 |
| Complexity | 772 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
ABOUT THIS PAGE
