
Synopsis
Synopsis
0
EU WC
0
KDMF
0
VMF
0
Australia

0
Listed Dossiers
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. 4306-cb
2. Chlorazepate
3. Clorazepate Dipotassium
4. Clorazepate Monopotassium
5. Clorazepic Acid
6. Dipotassium Chlorazepate
7. Tranxene
8. Tranxilium
1. Clorazepic Acid
2. Chlorazepate
3. 23887-31-2
4. 7-chloro-2,3-dihydro-2,2-dihydroxy-5-phenyl-1h-1,4-benzodiazepine-3-carboxylic Acid
5. 7-chloro-2-oxo-5-phenyl-1,3-dihydro-1,4-benzodiazepine-3-carboxylic Acid
6. 149128-44-9
7. 7-chloro-2-oxo-5-phenyl-2,3-dihydro-1h-1,4-benzodiazepine-3-carboxylic Acid
8. 1h-1,4-benzodiazepine-3-carboxylic Acid, 7-chloro-2,3-dihydro-2-oxo-5-phenyl-
9. 4306-cb Free Acid
10. Chebi:3761
11. D51wo0g0l4
12. Abbott-35616 Free Acid
13. Cchlorazepic Acid
14. Clorazepic Acid [ban]
15. Dea No. 2768
16. 7-chloro-2,3-dihydro-2-oxo-5-phenyl-1h-1,4-benzodiazepine-3-carboxylic Acid
17. Hsdb 3041
18. 4306 Cb
19. Einecs 245-926-8
20. Unii-d51wo0g0l4
21. Tranxene®
22. (chloromethyl)(dimethyl)(tridecyloxy)silane
23. Clorazepate [vandf]
24. Clorazepic Acid [mi]
25. Clorazepic Acid [hsdb]
26. Gtpl7548
27. Schembl1649064
28. Chembl1213252
29. Clorazepic Acid [mart.]
30. Schembl21244066
31. Clorazepic Acid [who-dd]
32. Dtxsid20863674
33. 23887-31-2 (free Acid)
34. Db00628
35. C06921
36. Q418850
37. 149128-42-7
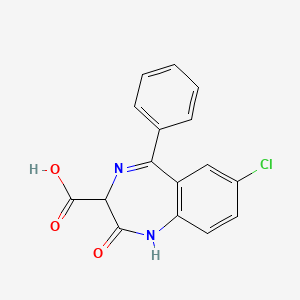
| Molecular Weight | 314.72 g/mol |
|---|---|
| Molecular Formula | C16H11ClN2O3 |
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 314.0458199 g/mol |
| Monoisotopic Mass | 314.0458199 g/mol |
| Topological Polar Surface Area | 78.8 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 488 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Clorazepate dipotassium |
| PubMed Health | Clorazepate (By mouth) |
| Drug Classes | Antianxiety, Anticonvulsant |
| Drug Label | The compound occurs as a fine, light yellow, practically odorless powder. It is insoluble in the common organic solvents, but very soluble in water. Aqueous solutions are unstable, clear, light yellow, and alkaline.Clorazepate dipotassium tablets con... |
| Active Ingredient | Clorazepate dipotassium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 3.75mg; 7.5mg; 15mg |
| Market Status | Prescription |
| Company | Ranbaxy; Taro; Mylan |
| 2 of 6 | |
|---|---|
| Drug Name | Gen-xene |
| PubMed Health | Clorazepate (By mouth) |
| Drug Classes | Antianxiety, Anticonvulsant |
| Drug Label | Chemically, TRANXENE is a benzodiazepine. The empirical formula is C16H11ClK2N2O4; the molecular weight is 408.92; 1H-1, 4-Benzodiazepine-3-carboxylic acid, 7-chloro-2,3-dihydro-2-oxo-5-phenyl-, potassium salt compound with potassium hydroxide (1:1)... |
| Active Ingredient | Clorazepate dipotassium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 7.5mg; 15mg; 3.75mg |
| Market Status | Prescription |
| Company | Alra |
| 3 of 6 | |
|---|---|
| Drug Name | Tranxene |
| Active Ingredient | Clorazepate dipotassium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 7.5mg; 15mg; 3.75mg |
| Market Status | Prescription |
| Company | Recordati Rare |
| 4 of 6 | |
|---|---|
| Drug Name | Clorazepate dipotassium |
| PubMed Health | Clorazepate (By mouth) |
| Drug Classes | Antianxiety, Anticonvulsant |
| Drug Label | The compound occurs as a fine, light yellow, practically odorless powder. It is insoluble in the common organic solvents, but very soluble in water. Aqueous solutions are unstable, clear, light yellow, and alkaline.Clorazepate dipotassium tablets con... |
| Active Ingredient | Clorazepate dipotassium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 3.75mg; 7.5mg; 15mg |
| Market Status | Prescription |
| Company | Ranbaxy; Taro; Mylan |
| 5 of 6 | |
|---|---|
| Drug Name | Gen-xene |
| PubMed Health | Clorazepate (By mouth) |
| Drug Classes | Antianxiety, Anticonvulsant |
| Drug Label | Chemically, TRANXENE is a benzodiazepine. The empirical formula is C16H11ClK2N2O4; the molecular weight is 408.92; 1H-1, 4-Benzodiazepine-3-carboxylic acid, 7-chloro-2,3-dihydro-2-oxo-5-phenyl-, potassium salt compound with potassium hydroxide (1:1)... |
| Active Ingredient | Clorazepate dipotassium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 7.5mg; 15mg; 3.75mg |
| Market Status | Prescription |
| Company | Alra |
| 6 of 6 | |
|---|---|
| Drug Name | Tranxene |
| Active Ingredient | Clorazepate dipotassium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 7.5mg; 15mg; 3.75mg |
| Market Status | Prescription |
| Company | Recordati Rare |
FOR SYMPTOMATIC RELIEF OF ANXIETY ASSOCIATED WITH NEUROSIS, PSYCHONEUROSES WITH SYMPTOMS OF ANXIETY, & AS ADJUNCT IN DISEASE STATES IN WHICH ANXIETY IS PROMINENT FEATURE. /DIPOTASSIUM SALT/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1010
RESULTS SUGGEST THAT CLORAZEPATE DIPOTASSIUM WHEN ADDED TO STD REGIMEN IN LOW DOSES, IS USEFUL IN TREATMENT OF INTRACTABLE MINOR SEIZURES. /CLORAZEPATE DIPOTASSIUM/
BOOKER HE; J AM MED ASSOC 229 (JUL): 552-5 (1974)
RESULTS SHOWED NO DIFFERENCE BETWEEN THE TWO & RESPONSE DID NOT DIFFER ACCORDING TO TYPE OF ANXIETY--PSYCHOLOGICAL OR SOMATIC. /FROM ABSTRACT/
PMID:23489 BURROW ET AL; MED J AUST 2 (OCT): 525-8 (1977)
Management of alcohol withdrawal /Clorazepate dipotassium from table/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 357
For more Therapeutic Uses (Complete) data for CLORAZEPATE (8 total), please visit the HSDB record page.
THIS DRUG SHOULD NOT BE DISCONTINUED ABRUPTLY, ALTHOUGH NO SERIOUS ADVERSE EFFECTS WERE OBSERVED UPON ABRUPT DISCONTINUATION OF 6 WK COURSE OF 120 MG DAILY. LONG-TERM THERAPY WITH LARGER THAN USUAL DOSES MAY RESULT IN PSYCHIC & PHYSICAL DEPENDENCE. /CLORAZEPATE DIPOTASSIUM SALT/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 414
CLORAZEPATE POTASSIUM REQUIRES SAME WARNINGS & PRECAUTIONS REGARDING USE WITH OTHER DRUGS, USE IN HYPERSENSITIVE INDIVIDUALS, USE DURING PREGNANCY & IN YOUNG CHILDREN, USE IN ELDERLY & EXCESSIVELY DEPRESSED PATIENT ... AS WITH OTHER BENZODIAZEPINES ... . /CLORAZEPATE DIPOTASSIUM/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1010
CLORAZEPATE POTASSIUM REQUIRES ... PRECAUTIONS ... IN PATIENT WITH IMPAIRED RENAL OR HEPATIC FUNCTION, & USE IN PATIENT WITH HISTORY OF DRUG ADDICTION AS WITH OTHER BENZODIAZEPINES ... . /CLORAZEPATE DIPOTASSIUM/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1010
PATIENTS RECEIVING ANY BENZODIAZEPINE CMPD SHOULD BE WARNED TO AVOID ALCOHOL, SINCE CONCURRENT INGESTION COULD RESULT IN AN IMPAIRMENT OF THEIR ABILITY TO DRIVE AUTOMOBILE OR OPERATE HAZARDOUS MACHINERY. /BENZODIAZEPINE CMPD/
Evaluations of Drug Interactions. 2nd ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1976, 1978., p. 47
For more Drug Warnings (Complete) data for CLORAZEPATE (10 total), please visit the HSDB record page.
For the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. Also used as adjunctive therapy in the management of partial seizures and for the symptomatic relief of acute alcohol withdrawal.
Clorazepate is a member of the group of drugs called benzodiazepines. Pharmacologically, clorazepate has the characteristics of the benzodiazepines. It has depressant effects on the central nervous system. The primary metabolite, nordiazepam, quickly appears in the blood stream. Studies in healthy men have shown that clorazenate has depressant effects on the central nervous system. Since orally administered clorazepate dipotassium is rapidly decarboxylated to form nordiazepam, there is essentially no circulating parent drug.
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
Absorption
Rapidly absorbed following oral administration (bioavailability is 91%).
Route of Elimination
The drug is metabolized in the liver and excreted primarily in the urine.
PRIMARY METABOLITE, NORDIAZEPAM, REACHES PEAK LEVELS IN PLASMA IN 1 HR; PLASMA HALF-LIFE IS ABOUT 24 HR.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1010
PLASMA HALF-LIFE ELIMINATION COEF, CONCN, & APPARENT VOL OF DISTRIBUTION CALCULATED & MEAN VALUES WERE 53 HR, 0.0147 HR-1, 884 NG/ML, & 1.13 L/KG.
POST ET AL; PSYCHOPHARMACOLOGY (BERLIN) 53 (2): 105-9 (1977)
TIME REQUIRED TO REACH PEAK SERUM CONCN OF N-DIMETHYLDIAZEPAM, METABOLITE OF CHLORAZEPATE, WAS 80 MIN. MEAN HALF-LIFE 62 HR. DATA FOR OTHERS GIVEN.
WRETLIND M ET AL; ACTA PHARMACOL TOXICOL, SUPPL 40 (1): 28-39 (1977)
WHEN ADMIN IM, CLORAZEPATE WAS RAPIDLY ABSORBED & PEAK CONCN REACHED IN 2 HR. ABSORPTION HALF-LIFE WAS 0.77 HR IN PREGNANT WOMEN & 0.56 HR IN NONPREGNANT WOMEN.
PMID:37089 REY ET AL; EUR J CLIN PHARMACOL 15 (3): 175-80 (1979)
For more Absorption, Distribution and Excretion (Complete) data for CLORAZEPATE (12 total), please visit the HSDB record page.
The drug is metabolized in the liver and excreted primarily in the urine. The primary metabolite, nordiazepam, is further metabolized by hydroxylation. The major urinary metabolite is conjugated oxazepam (3-hydroxynordiazepam), and smaller amounts of conjugated p-hydroxynordiazepam and nordiazepam are also found in the urine.
CHLORAZEPATE IS PROBABLY CONVERTED TO N-DIMETHYL DIAZEPAM, WHICH IS THEN OXIDIZED TO OXAZEPAM. /CHLORAZEPATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 189
PRIMARY METABOLITE /IS/ NORDIAZEPAM ... .
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1010
Following oral administration of clorazepate dipotassium, it appears that most of the drug is rapidly decarboxylated in the GI tract and is absorbed as desmethyldiazepam (nordiazepam). The rate of decarboxylation of clorazepate decreases as gastric pH increases. /Clorazepate dipotassium/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1331
Clorazepate active metabolites in liver are desmethyldiazepam, and oxazepam; active substances in blood include desmethyldiazepam. /From table/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 582
For more Metabolism/Metabolites (Complete) data for CLORAZEPATE (8 total), please visit the HSDB record page.
The serum half-life is about 2 days. Nordiazepam, the primary metabolite, quickly appears in the blood and is eliminated from the plasma with an apparent half-life of about 40 to 50 hours.
PLASMA HALF-LIFE ... 53 HR ... .
POST ET AL; PSYCHOPHARMACOLOGY (BERLIN) 53 (2): 105-9 (1977)
PRIMARY METABOLITE, NORDIAZEPAM, ... PLASMA HALF-LIFE IS ABOUT 24 HR.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1010
N-DIMETHYLDIAZEPAM, METABOLITE OF CHLORAZEPATE: MEAN HALF-LIFE 62 HR.
WRETLIND M ET AL; ACTA PHARMACOL TOXICOL, SUPPL 40 (1): 28-39 (1977)
CLORAZEPATE ADMIN IM TO PREGNANT & NONPREGNANT WOMEN. ELIMINATION HALF-LIFE WERE 1.3 HR IN PREGNANT WOMEN & 2.0 HR IN NONPREGNANT WOMEN. ITS METABOLITE, NORDIAZEPAM, REACHED PEAK CONCN WITHIN 12 HR. HALF-LIFE OF ELIMINATION FOR METABOLITE WAS 180 IN PREGNANT & 60 HR IN NONPREGNANT WOMEN.
PMID:37089 REY ET AL; EUR J CLIN PHARMACOL 15 (3): 175-80 (1979)
Elimination half-lives for metabolites: desmethyldiazepam (30-200 hr) and oxazepam (3-21 hr)
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1331
Benzodiazepines bind nonspecifically to benzodiazepine receptors BNZ1, which mediates sleep, and BNZ2, which affects affects muscle relaxation, anticonvulsant activity, motor coordination, and memory. As benzodiazepine receptors are thought to be coupled to gamma-aminobutyric acid-A (GABAA) receptors, this enhances the effects of GABA by increasing GABA affinity for the GABA receptor. Binding of the inhibitory neurotransmitter GABA to the site opens the chloride channel, resulting in a hyperpolarized cell membrane that prevents further excitation of the cell.
The effects of benzodiazepines on the waking EEG resemble those of other sedative-hypnotic drugs. Alpha activity is decreased, and there is an increase in low-voltage fast activity, especially beta activity. /Benzodiazepines/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 349
Acticonvulsant actions of the benzodiazepines, as well as other effects that occur at nonsedating doses, result in large part from their ability to enhance GABA-induced increases in the conductance of chloride. /Benzodiazepines/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 454
Benzodiazepines appear to produce their sedative, hypnotic, anxiolytic, and anticonvulsant actions by binding to specific pharmacologic receptors in the central nervous system. Benzodiazepine receptors are most concentrated in the cerebral cortex but are also found in the cerebellum, amygdala, hippocampus, hypothalamus, and spinal cord. Receptors have also been identified outside CNS, although peripheral receptors do not appear to have significant physiologic effects. The molecular receptor for the benzodiazepine molecule is a glycoprotein located on the lipid membranes of both neural and glial cells. It is speculated that there are at least two classes of benzodiazepine receptor believed to mediate different specific effects. Type I receptors predominate in the cerebelar cortex, and both type I and type II receptors are found in the cerebral cortex and hippocampus. Type I rceptors are postulated to mediate anxiolytic effects, and type II receptors mediate the sedative and other actions of benzodiazepines. No endogenous ligand or neurotransmitter for these receptors has been identified. /Benzodiazepines/
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 806

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
11
PharmaCompass offers a list of Clorazepate Dipotassium API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Clorazepate Dipotassium manufacturer or Clorazepate Dipotassium supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Clorazepate Dipotassium manufacturer or Clorazepate Dipotassium supplier.
PharmaCompass also assists you with knowing the Clorazepate Dipotassium API Price utilized in the formulation of products. Clorazepate Dipotassium API Price is not always fixed or binding as the Clorazepate Dipotassium Price is obtained through a variety of data sources. The Clorazepate Dipotassium Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Clorazepate Dipotassium manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Clorazepate Dipotassium, including repackagers and relabelers. The FDA regulates Clorazepate Dipotassium manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Clorazepate Dipotassium API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Clorazepate Dipotassium manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Clorazepate Dipotassium supplier is an individual or a company that provides Clorazepate Dipotassium active pharmaceutical ingredient (API) or Clorazepate Dipotassium finished formulations upon request. The Clorazepate Dipotassium suppliers may include Clorazepate Dipotassium API manufacturers, exporters, distributors and traders.
click here to find a list of Clorazepate Dipotassium suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Clorazepate Dipotassium DMF (Drug Master File) is a document detailing the whole manufacturing process of Clorazepate Dipotassium active pharmaceutical ingredient (API) in detail. Different forms of Clorazepate Dipotassium DMFs exist exist since differing nations have different regulations, such as Clorazepate Dipotassium USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Clorazepate Dipotassium DMF submitted to regulatory agencies in the US is known as a USDMF. Clorazepate Dipotassium USDMF includes data on Clorazepate Dipotassium's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Clorazepate Dipotassium USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Clorazepate Dipotassium suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Clorazepate Dipotassium Drug Master File in Japan (Clorazepate Dipotassium JDMF) empowers Clorazepate Dipotassium API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Clorazepate Dipotassium JDMF during the approval evaluation for pharmaceutical products. At the time of Clorazepate Dipotassium JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Clorazepate Dipotassium suppliers with JDMF on PharmaCompass.
A Clorazepate Dipotassium CEP of the European Pharmacopoeia monograph is often referred to as a Clorazepate Dipotassium Certificate of Suitability (COS). The purpose of a Clorazepate Dipotassium CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Clorazepate Dipotassium EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Clorazepate Dipotassium to their clients by showing that a Clorazepate Dipotassium CEP has been issued for it. The manufacturer submits a Clorazepate Dipotassium CEP (COS) as part of the market authorization procedure, and it takes on the role of a Clorazepate Dipotassium CEP holder for the record. Additionally, the data presented in the Clorazepate Dipotassium CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Clorazepate Dipotassium DMF.
A Clorazepate Dipotassium CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Clorazepate Dipotassium CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Clorazepate Dipotassium suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Clorazepate Dipotassium as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Clorazepate Dipotassium API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Clorazepate Dipotassium as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Clorazepate Dipotassium and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Clorazepate Dipotassium NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Clorazepate Dipotassium suppliers with NDC on PharmaCompass.
Clorazepate Dipotassium Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Clorazepate Dipotassium GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Clorazepate Dipotassium GMP manufacturer or Clorazepate Dipotassium GMP API supplier for your needs.
A Clorazepate Dipotassium CoA (Certificate of Analysis) is a formal document that attests to Clorazepate Dipotassium's compliance with Clorazepate Dipotassium specifications and serves as a tool for batch-level quality control.
Clorazepate Dipotassium CoA mostly includes findings from lab analyses of a specific batch. For each Clorazepate Dipotassium CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Clorazepate Dipotassium may be tested according to a variety of international standards, such as European Pharmacopoeia (Clorazepate Dipotassium EP), Clorazepate Dipotassium JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Clorazepate Dipotassium USP).
