
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Colimycin
2. Colisticin
3. Colistin
4. Colistin Sulfate
5. Coly-mycin
6. Sulfate, Colistin
7. Totazina
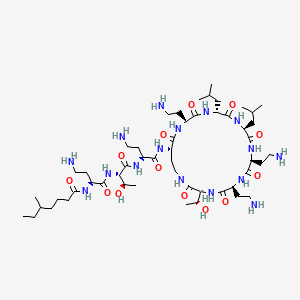
1. Colistin
2. 1066-17-7
3. N-[(2s)-4-amino-1-[[(2s,3r)-1-[[(2s)-4-amino-1-oxo-1-[[(3s,6s,9s,12s,15r,18s,21s)-6,9,18-tris(2-aminoethyl)-3-[(1r)-1-hydroxyethyl]-12,15-bis(2-methylpropyl)-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptazacyclotricos-21-yl]amino]butan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1-oxobutan-2-yl]-5-methylheptanamide
4. Colobreathe
5. Promixin
6. Colistin,(s)
7. Chembl499783
8. Schembl1979092
9. Gtpl10794
10. Colistin Sulfate, >19000 Iu/mg
11. Ab01566924_01
12. 066c177
13. Q418946
14. Sr-01000872582
15. Sr-01000872582-1
16. N-[(1s)-3-amino-1-[[(1s,2r)-1-[[(1s)-3-amino-1-[[(3s,6s,9s,12s,15r,18s,21s)-6,9,18-tris(2-aminoethyl)-3-[(1r)-1-hydroxyethyl]-12,15-diisobutyl-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptazacyclotricos-21-yl]carbamoyl]propyl]carbamoyl]-2-hydroxy-propyl]carbamoyl]propyl]-5-methyl-heptanamide
17. N-[(1s)-3-amino-1-{[(1s,2r)-1-{[(1s)-3-amino-1-{[(3s,6s,9s,12s,15r,18s,21s)-6,9,18-tris(2-aminoethyl)-3-[(1r)-1-hydroxyethyl]-12,15-bis(2-methylpropyl)-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptaazacyclotricosan-21-yl]carbamoyl}propyl]carbamoyl}-2-hydroxypropyl]carbamoyl}propyl]-5-methylheptanamide
| Molecular Weight | 1155.4 g/mol |
|---|---|
| Molecular Formula | C52H98N16O13 |
| XLogP3 | -3.3 |
| Hydrogen Bond Donor Count | 18 |
| Hydrogen Bond Acceptor Count | 18 |
| Rotatable Bond Count | 28 |
| Exact Mass | 1154.74992724 g/mol |
| Monoisotopic Mass | 1154.74992724 g/mol |
| Topological Polar Surface Area | 491 Ų |
| Heavy Atom Count | 81 |
| Formal Charge | 0 |
| Complexity | 2050 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 12 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antibiotics, Peptide
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
THERAPEUTIC INDICATIONS FOR COLISTIN ARE ESSENTIALLY SAME AS THOSE FOR POLYMYXIN B. INFECTIONS OF CERTAIN TYPES CAUSED BY PSEUD AERUGINOSA ARE ESPECIALLY SUSCEPTIBLE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1233
PRIMARY USE OF POLYMYXIN B IS FOR TREATMENT OF INFECTIONS CAUSED BY GRAM-NEGATIVE BACTERIA, ESPECIALLY PSEUDOMONAS. ...IS EFFECTIVE IN TREATMENT OF URINARY TRACT INFECTIONS CAUSED BY PSEUDOMONAS OR OTHER GRAM-NEGATIVE BACILLI RESISTANT TO OTHER ANTIMICROBIAL AGENTS... /POLYMYXIN B/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1232
/POLYMYXIN B & COLISTIMETHATE SODIUM/...RECOMMENDED FOR TREATING PERITONITIS & PNEUMONIA, BUT SOME AUTHORITIES QUESTION THEIR EFFECTIVENESS FOR THESE PURPOSES. /COLISTIMETHATE SODIUM/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 734
For more Therapeutic Uses (Complete) data for COLISTIN (11 total), please visit the HSDB record page.
ADVERSE REACTIONS TO COLISTIMETHATE HAVE BEEN NOTED IN 20% OF PT GIVEN THE DRUG; THEY ARE GENERALLY REVERSIBLE... /COLISTIMETHATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1233
COLISTIMETHATE SODIUM SHOULD NOT BE ADMIN INTRATHECALLY. /COLISTIMETHATE SODIUM/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 734
...NOT INDICATED FOR INFECTIONS CAUSED BY PROTEUS OR NEISSERIA SPECIES. /SODIUM COLISTIMETHATE/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 394
POSSIBLE EMBRYOTOXIC & TERATOGENIC EFFECTS HAVE BEEN REPORTED WHEN COLISTIMETHATE SODIUM WAS ADMINISTERED TO PREGNANT RABBITS. /NA COLISTIMETHATE/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 395
For more Drug Warnings (Complete) data for COLISTIN (13 total), please visit the HSDB record page.
Colobreathe is indicated for the management of chronic pulmonary infections due to Pseudomonas aeruginosa in patients with cystic fibrosis (CF) aged six years and older.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01XB01
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07A - Intestinal antiinfectives
A07AA - Antibiotics
A07AA10 - Colistin
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01X - Other antibacterials
J01XB - Polymyxins
J01XB01 - Colistin
/COLISTIMETHATE SODIUM/...IS EXCRETED IN DOG URINE TO MUCH GREATER EXTENT THAN SIMPLE SULFATE FORM, & THEORETICALLY SHOULD BE BETTER IN URINARY INFECTIONS.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 130
DRUG PASSES FROM MATERNAL TO FETAL CIRCULATION. PREMATURE INFANTS INJECTED WITH 1 MG/KG DO NOT DEVELOP EFFECTIVE PLASMA CONCN OF ANTIBIOTIC; WITH DOSE OF 2 MG/KG, PEAK VALUE OF ABOUT 5 UG/ML IS REACHED IN 30 MIN. /COLISTIMETHATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1233
PLASMA CONCN ARE HIGHER IN PERSONS WITH RENAL INSUFFICIENCY & ARE RELATED TO DEGREE OF RENAL DYSFUNCTION. COLISTIMETHATE IS EXCRETED MAINLY BY GLOMERULAR FILTRATION. URINE CONCN EXCEED 200 UG/ML DURING FIRST 2 HR AFTER USUAL IM DOSE. /COLISTIMETHATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1233
DRUG IS EXCRETED MORE RAPIDLY IN CHILDREN THAN IN ADULTS. COLISTIMETHATE DOES NOT GAIN ACCESS TO CEREBROSPINAL FLUID, EVEN WHEN MENINGES ARE INFLAMED. /COLISTIMETHATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1233
For more Absorption, Distribution and Excretion (Complete) data for COLISTIN (8 total), please visit the HSDB record page.
...hydrolyzed in vivo to colistin and possibly other metabolites with fewer substituted amino groups...
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 394
AFTER IV INJECTION RABBITS, 75% DOSE EXCRETED IN URINE UNCHANGED. SMALL AMT FOUND IN BILE. COLISTIN-N-GLUCURONIDE FOUND IN URINE (1.7% OF DOSE) & BILE (6.7% OF DOSE). /SODIUM COLISTIN METHANE SULFONATE/
ABE ET AL; CHEMOTHERAPY (TOKYO) 24(8) 1592-1596 (1976)
IM INJECTION OF 150 MG OF COLISTIMETHATE IN ADULTS PRODUCE PEAK PLASMA CONCN OF 6 UG/ML @ 2 HR; THIS DECLINES WITH HALF-TIME OF 2 HR. SAME QUANTITY GIVEN IV YIELDS MAXIMAL PLASMA CONCN OF 18 UG/ML; THIS FALLS TO ABOUT 0.4 UG/ML @ 12 HR. /COLISTIMETHATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1233
POLYMYXIN B IS SURFACE-ACTIVE AGENT... CONTAINING LIPOPHILIC & LIPOPHOBIC GROUPS SEPARATED WITHIN MOLECULE. /POLYMYXIN B/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1144
PERMEABILITY OF THE BACTERIAL MEMBRANE CHANGES IMMEDIATELY ON CONTACT WITH DRUG. SENSITIVITY TO POLYMYXIN B APPARENTLY IS RELATED TO THE PHOSPHOLIPID CONTENT OF THE CELL WALL-MEMBRANE COMPLEX. /POLYMYXIN B/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1144
Colistin acts like a cationic detergent and binds to and damages the bacterial cytoplasmic membrane of susceptible bacteria. Damage to the bacterial cytoplasmic membrane alters the osmotic barrier of the membrane and causes leakage of essential intracellular metabolites and nucleosides.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 394

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
60
PharmaCompass offers a list of Colistin Sulfate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Colistin Sulfate manufacturer or Colistin Sulfate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Colistin Sulfate manufacturer or Colistin Sulfate supplier.
PharmaCompass also assists you with knowing the Colistin Sulfate API Price utilized in the formulation of products. Colistin Sulfate API Price is not always fixed or binding as the Colistin Sulfate Price is obtained through a variety of data sources. The Colistin Sulfate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Colistin Sulfate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Colistin Sulfate, including repackagers and relabelers. The FDA regulates Colistin Sulfate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Colistin Sulfate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Colistin Sulfate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Colistin Sulfate supplier is an individual or a company that provides Colistin Sulfate active pharmaceutical ingredient (API) or Colistin Sulfate finished formulations upon request. The Colistin Sulfate suppliers may include Colistin Sulfate API manufacturers, exporters, distributors and traders.
click here to find a list of Colistin Sulfate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Colistin Sulfate DMF (Drug Master File) is a document detailing the whole manufacturing process of Colistin Sulfate active pharmaceutical ingredient (API) in detail. Different forms of Colistin Sulfate DMFs exist exist since differing nations have different regulations, such as Colistin Sulfate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Colistin Sulfate DMF submitted to regulatory agencies in the US is known as a USDMF. Colistin Sulfate USDMF includes data on Colistin Sulfate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Colistin Sulfate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Colistin Sulfate suppliers with USDMF on PharmaCompass.
A Colistin Sulfate CEP of the European Pharmacopoeia monograph is often referred to as a Colistin Sulfate Certificate of Suitability (COS). The purpose of a Colistin Sulfate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Colistin Sulfate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Colistin Sulfate to their clients by showing that a Colistin Sulfate CEP has been issued for it. The manufacturer submits a Colistin Sulfate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Colistin Sulfate CEP holder for the record. Additionally, the data presented in the Colistin Sulfate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Colistin Sulfate DMF.
A Colistin Sulfate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Colistin Sulfate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Colistin Sulfate suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Colistin Sulfate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Colistin Sulfate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Colistin Sulfate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Colistin Sulfate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Colistin Sulfate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Colistin Sulfate suppliers with NDC on PharmaCompass.
Colistin Sulfate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Colistin Sulfate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Colistin Sulfate GMP manufacturer or Colistin Sulfate GMP API supplier for your needs.
A Colistin Sulfate CoA (Certificate of Analysis) is a formal document that attests to Colistin Sulfate's compliance with Colistin Sulfate specifications and serves as a tool for batch-level quality control.
Colistin Sulfate CoA mostly includes findings from lab analyses of a specific batch. For each Colistin Sulfate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Colistin Sulfate may be tested according to a variety of international standards, such as European Pharmacopoeia (Colistin Sulfate EP), Colistin Sulfate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Colistin Sulfate USP).
