

Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
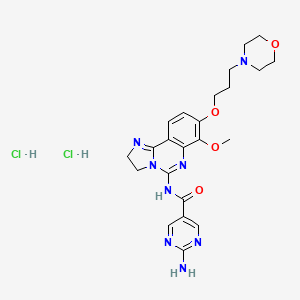

| Molecular Weight | 553.4 g/mol |
|---|---|
| Molecular Formula | C23H30Cl2N8O4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 8 |
| Exact Mass | 552.1767069 g/mol |
| Monoisotopic Mass | 552.1767069 g/mol |
| Topological Polar Surface Area | 140 A^2 |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 796 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
