
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
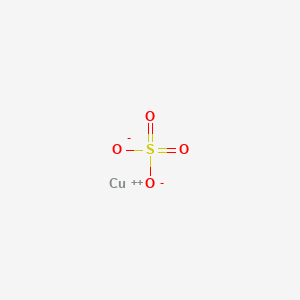

1. Blue Vitriol
2. Copper Sulfate
3. Sulfate, Copper
4. Sulfate, Cupric
5. Vitriol, Blue
1. Copper Sulfate
2. 7758-98-7
3. Copper(ii) Sulfate
4. Copper Sulphate
5. Cupric Sulfate Anhydrous
6. Copper(2+) Sulfate
7. Copper(ii) Sulfate, Anhydrous
8. Copper Monosulfate
9. Cupricsulfate
10. Blue Stone
11. Copper(2+) Sulphate
12. Copper Ii Sulfate
13. Copper Sulfate (1:1)
14. Sulfuric Acid Copper(2+) Salt (1:1)
15. Copper;sulfate
16. Cuso4
17. Copper(ii) Sulphate
18. Coppersulfate
19. Copper(2+) Sulfate (1:1)
20. 10124-44-4
21. Copper Sulfate, Anhydrous
22. Copper Monosulphate
23. 18939-61-2
24. Sulfuric Acid, Copper(2+) Salt
25. Cupric Sulphate Anhydrous
26. Cupric Sulfate, Anhydrous
27. Cupric Sulphate, Anhydrous
28. Kuw2q3u1vv
29. Copper(ii) Sulfate Solution
30. 1332-14-5
31. Hylinec
32. Trinagle
33. Delcup
34. Sulfuric Acid, Coppersalt (8ci,9ci)
35. Monocopper Sulfate
36. Incracide 10a
37. Bcs Copper Fungicide
38. Blue Copper (van)
39. Bonide Root Destroyer
40. Copper Sulfate Powder
41. Kupfersulfat [german]
42. All Clear Root Destroyer
43. Snow Crystal Copper Sulfate
44. Sulfate De Cuivre [french]
45. Aqua Maid Permanent Algaecide
46. Ccris 3665
47. Hsdb 916
48. Granular Crystals Copper Sulfate
49. Copper (ii) Sulphate
50. Mac 570
51. Bluestone Copper Sulfate
52. Tobacco States Brand Copper Sulfate
53. Einecs 231-847-6
54. Phelps Triangle Brand Copper Sulfate
55. Unii-kuw2q3u1vv
56. Mfcd00010981
57. Nsc 57630
58. Sulfuric Acid, Copper Salt
59. Copper (ii) Sulfate Anhydrous
60. Sa-50 Brand Copper Sulfate Granular Crystals
61. Aquatronics Snail-a-cide Dri-pac Snail Powder
62. Copper(ii)sulphate
63. Copper(ii)-sulfate
64. Einecs 242-692-9
65. Copper (as Sulfate)
66. Copper Sulfate Chelate
67. Cuso4 Copper Sulphate
68. Copper (11) Sulfate
69. Copper( Cento) Sulfate
70. Sulfuric Acid, Copper(2+) Salt (1:?)
71. Cupric Sulfate,anhydrous
72. Bluestone, Cupric Sulfate
73. Copper(ii) Tetraoxosulfate
74. Cupric Sulfate [mi]
75. Copper Sulfate [inci]
76. Cupric Sulfate [hsdb]
77. Sulfuric Acid Copper(2+)salt
78. Copper Sulfate [who-dd]
79. Dtxsid6034479
80. Chebi:23414
81. Copper Sulphate (1:1)
82. Cupric Sulfate Anhydrous [ii]
83. Akos015902901
84. Copper(2+) Sulphate (1:1)
85. Db06778
86. Cupric Sulfate,anhydrous [vandf]
87. Bp-20356
88. Fishertab™ Ct-37 Kjeldahl Tablets
89. Fishertab™ Ct-50 Kjeldahl Tablets
90. Fishertab™ Tt-35 Kjeldahl Tablets
91. Fishertab™ Tt-43 Kjeldahl Tablets
92. Fishertab™ Tt-50 Kjeldahl Tablets
93. Fishertab™ Tt-57 Kjeldahl Tablets
94. Fishertab™ Ct-auto Kjeldahl Tablets
95. Ft-0624048
96. C18713
97. Copper Sulfate, Anhydrous [ep Impurity]
98. Cupric Sulfate Anhydrous [usp Monograph]
99. Cupric Sulphate, Copper Sulphate, Cupric Sulfate
100. A923422
101. Q107184
102. Sr-01000944582
103. Sr-01000944582-1
| Molecular Weight | 159.61 g/mol |
|---|---|
| Molecular Formula | CuO4S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 158.881327 g/mol |
| Monoisotopic Mass | 158.881327 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antidotes; Emetics; Fungicides, Industrial
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/SRP: EXTERNAL/ ANTIDOTE FOR WHITE PHOSPHORUS POISONING.
SRI
/SRP: FORMER USE/ A 0.1% soln of copper sulfate has been used for gastric lavage in phosphorus poisoning; it must be removed promptly to avoid copper poisoning. Topical application of a 1% soln is of value for phosphorus burns of the skin.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 931
EXPT USE: IN EXPT WITH RATS TO FIND SIMPLE EFFICIENT ANTIDOTE FOR PHOSPHORUS BURNS, USE OF 5% COPPER SULFATE WAS HIGHLY TOXIC. SOLN OF 5% SODIUM BICARBONATE WITH 1% HYDROXYETHYL-CELLULOSE, 3% COPPER SULFATE & LAURYL SULFATE NEUTRALIZES PROCESS OF BURNING PHOSPHORUS.
PMID:5047150 BEN-HUR N ET AL; BR J PLAST SURG 25 (3): 245-9 (1972)
For more Therapeutic Uses (Complete) data for COPPER(II) SULFATE (11 total), please visit the HSDB record page.
... Its routine use as an emetic is not recommended, because of the potential toxicity of improperly prepared soln and the hazards attending the use of large, corrosive doses.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 748
Overdose may be poisonous (enteritis, hepatitis, nephritis).
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 136
MAY CAUSE DRAMATIC INCR IN MORTALITY OF TURKEYS GIVEN BLACKHEAD CONTROL DRUGS CONTAINING ARSENIC & EXPOSED TO BLACKHEAD.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 136
CUPRIC ... SULFATE /AS EMETIC/ OFTEN IS EFFECTIVE, BUT POTENTIAL HEMOLYTIC & RENAL TOXICITY IS TOO GREAT TO RECOMMEND USE.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 1436
Elemental use in copper deficiency Copper and copper containing compounds are broadly used in medical practice. Metallic copper is used already for many years in dental fillings and in copper intrauterine devices (IUD) for reversible contraception. Ointments containing copper, which release copper ions that are absorbed by the skin in the management of cramps, disturbances of renal function, peripheral, venous hypostatic circulatory disturbances, rheumatic disease and swelling associated with trauma. There are also cosmetic facial creams containing copper as their main active ingredient.
Copper is an essential mineral that plays a key role in many physiological processes, including angiogenesis, skin generation and expression and stabilization of skin proteins. Copper is found naturally in many food sources including meats, vegetables, and grains. Copper has potent biocidal properties and is used to eliminate bacteria, viruses and parasites,. Copper is one of the nine essential minerals for humans, as it plays an imperative role in various physiological pathways in basically all human tissue, as well as in the health of the dermis and epidermis. In addition to the above, copper is essential in wound healing, as it promotes angiogenesis and skin extracellular matrix formation and stabilization.
Emetics
Agents that cause vomiting. They may act directly on the gastrointestinal tract, bringing about emesis through local irritant effects, or indirectly, through their effects on the chemoreceptor trigger zone in the postremal area near the medulla. (See all compounds classified as Emetics.)
Antidotes
Agents counteracting or neutralizing the action of POISONS. (See all compounds classified as Antidotes.)
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AB - Antidotes
V03AB20 - Copper sulfate
Absorption
Primarily absorbed in the small intestine. Based on studies with radioactive isotopes of copper, most copper is absorbed from the stomach and duodenum of the gastrointestinal tract. Maximum blood copper levels are observed within 1 to 3 hours following oral administration, and about 50 percent of ingested copper was absorbed. Copper absorption is proposed to occur by two mechanisms, one energy- dependent and the other enzymatic. Factors that can interfere with copper absorption include competition for binding sites with zinc, interactions with molybdenum and sulfates, chelation with phytates, and inhibition by ascorbic acid (vitamin C). Copper absorbed from the gastrointestinal tract is transported rapidly to blood serum and deposited in the liver bound to metallothionein. From 20 to 60% of the dietary copper is absorbed.
Route of Elimination
This drug is 80% eliminated via the liver in bile. Minimal excretion by the kidney. Metabolism studies show that persons with daily intakes of 2-5 mg of copper per day absorbed 0.6 to 1.6 mg (32%), excreted 0.5 to 1.3 mg in the bile, passed 0.1 to 0.3 mg directly into the bowel, and excreted 0.01 to 0.06 mg in the urine. As the data indicate, urinary excretion plays a negligible role in copper clearance, and the main route of excretion is in the bile. Other nonsignificant excretory routes include saliva, sweat, menstrual flow, and excretion into the intestine from the blood.
Volume of Distribution
The body of a 70 kg healthy individual contains approximately 110 mg of copper, 50% of which is found in the bones and muscles, 15% in the skin, 15% in the bone marrow, 10% in the hepatic system, and 8% in the brain. The distribution of copper is affected by sex, age, and the amount of copper in the diet. Brain and liver have the highest tissue levels (about one-third of the total body burden), with lesser concentrations found in the heart, spleen, kidneys, and blood. The iris and choroid of the eye have very high copper levels. Erythrocyte copper levels are generally stable, however, plasma levels fluctuate widely in association with the synthesis and release of ceruloplasmin. Plasma copper levels during gestation may be 2-3 times levels measured before pregnancy, due to the increased synthesis of ceruloplasmin.
Effect of hydrogen ion (H+) concentration, water hardness, suspended solids, fish age, size, and species, acclimatization to copper, and levels of copper in food on poisoning of fish by copper sulfate used as a herbicide in freshwater ponds is discussed. Copper levels in muscle, kidney, and organs of rainbow trout were approximately 0.8-1.1, 2.0-2.3, and 115-150 mg/kg fresh weight, respectively, after 12 months intermittent exposure to various copper sulfate containing formulations 0.6, 2.0, and 100 mg/kg, respectively, in controls ... .
Bohl M, Wagner H; Fisch Umwelt 10(Beitr Fischtoxikol - Parasitol): 121-7 (1981)
Male rats were orally administered for 2, 5, and 11 days with 0.5 mmol/kg of copper cmpd. ... In the case of cupric carbonate, copper was much more distributed in the tissues, especially in the liver, than for copper sulfate. The copper level increased progresively in mitochondria lysosomal fractions of the liver in proportion to the period of administration. In the 105,000 g supernatant fraction, copper was distributed in the metallothionein fraction rather than in the superoxide dismutase fraction. The administration of copper cmpd resulted in an increase in the zinc level in the liver, kidney and spleen, preferentially in the metallothionein fraction of the liver, but it seemed to have little effect on iron metabolism.
Fujita M et al; Eisei Kagaku 30 (2): 69-78 (1984)
Maximum blood copper levels were observed within 1 to 3 hours following oral administration, and about 50 percent of ingested copper was absorbed. Copper absorption is believed to occur by two mechanisms, one energy- dependent and the other enzymatic. Factors that can interfere with copper absorption include competition for binding sites with zinc, interactions with molybdenum and sulfates, chelation with phytates, and inhibition by ascorbic acid. Copper absorbed from the intestine is transported quickly into blood serum and deposited in the liver bound to metallothionein. It is released and incorporated into ceruloplasmin, a copper-specific transport protein. The remaining copper in the serum binds to albumin or amino acids or is contained in the erythrocytes. About 80 percent of the absorbed copper is bound to liver metallothionein; the remainder is included into cytochrome c oxidase or sequestered by lysosomes.
The biological half-life of copper from the diet is 13-33 days with biliary excretion being the primary route of elimination.
This drug is an essential trace element for the functioning of many metalloenzymes including ceruloplasmin, ferroxidase II, lysyl oxidase, monoamine oxidase, Zn-copper superoxide dismutase, tyrosinase, dopamine--hydroxylase, and cytochrome-c-oxidase. It is involved in erythropoiesis & leukopoiesis, bone mineralization, elastin and collagen cross-linking, oxidative phosphorylation, catecholamine metabolism, melanin formation & antioxidant protection of cells. Cupric sulfate may also have a role in iron turnover, ascorbic acid metabolism, phospholipid metabolism, myelin formation, glucose homeostasis, and cellular immune defense. After the metal passes through the basolateral membrane it is transported to the liver, attached to serum albumin. The liver is the critical organ for the homeostasis of copper. The copper is then prepared for excretion through the bile or incorporation into various proteins. The transport of copper to the peripheral tissues is accomplished through the plasma attached to serum albumin, ceruloplasmin or low-molecular-weight complexes. In the dermis, copper promotes dermal fibroblasts proliferation, upregulates collagen (types I, II, and V) and elastin fiber components (elastin, fibrillins) production by fibroblasts, through the induction of TGF-, promotes heat shock protein-47, important for collagen fibril formation, serves as a cofactor of LOX enzyme required for extracellular matrix protein cross-linking, stabilizes the skin ECM once formed, as increased crosslinking of collagen and elastin matrices occurs in a copper dose dependant manner, serves as a cofactor of superoxide dismutase, an antioxidant enzyme in the skin, essential for protection against free radicals, inhibits cellular oxidative effects such as membrane damage and lipid peroxidation, acts as a cofactor of tyrosinase, a melanin biosynthesis essential enzyme responsible for skin and hair pigmentation. In reference to its role as a biocide, copper is an essential nutrient for many organisms. It acts as a cofactor in respiration, and therefore copper is required for aerobic metabolism. Accumulation of copper ions or intracellular release of free copper ions from proteins lead to cell damage. Copper catalyzes reactions that result in the production of hydroxyl radicals through the Fenton and Haber-Weiss reactions. The highly reactive oxygen intermediates lead to lipid peroxidation and oxidation of proteins. Free copper ions oxidize sulfhydryl groups, such as cysteine, in proteins or the cellular redox buffer glutathione. In particular, copper ions inactivate proteins by damaging Fe-S clusters in cytoplasmic hydratases.
A significant drop in metabolic reserves was noted in cupric sulfate treated snails. Free amino acid levels dropped and the lactate level increased. The effects of copper treatment on rates of metabolite oxidation and ammonia production in the presence of exogenously added alpha-ketoglutarate were evaluated. A 92% drop in alpha-ketoglutarate dehydrogenase, a 33% drop in alanine aminotransferase, and a 78% rise in glucose 6-phosphatase were recorded. Molluscicidal activity of copper was due to a metabolic block in the tricarboxylic acid cycle at the alpha-ketoglutarate level.
PMID:3559915 Babu GR, Rao PV; J Environ Pathol Toxicol Oncol 7 (3): 29-34 (1987)
ACUTE IV INFUSION OF 300 MG COPPER SULFATE IN CONSCIOUS SHEEP CAUSED AN INCREASE FROM 10.3 TO 22.5 TORR IN MEAN PULMONARY ARTERY PRESSURE & PULMONARY ARTERY WEDGE PRESSURE FROM 3.5 TO 7.6 TORR, WHEREAS SYSTEMIC ARTERIAL PRESSURE INCREASED FROM 95 TO 102 TORR. CARDIAC OUTPUT DECREASED FROM 4.7 TO 3.3 L/MIN. PULMONARY VASCULAR RESISTANCE & SYSTEMIC VASCULAR RESISTANCE INCREASED TO 320 & 160% OF BASE LINE, RESPECTIVELY. THIS PULMONARY HYPERTENSION WAS PRODUCED BY STIMULATION OF THE ALPHA-ADRENERGIC NERVOUS SYSTEM.
PMID:6271712 AHMED T ET AL; J APPL PHYSIOL 51 (5): 1204-13 (1981)
Related Excipient Companies

Excipients by Applications
Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?