
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Calcium, Rosuvastatin
2. Crestor
3. Rosuvastatin Calcium
4. Zd 4522
5. Zd4522
1. 287714-41-4
2. Creston
3. Rosuvastatin Calcium
4. Zd4522
5. Rosuvastatin [inn]
6. X-plended
7. Zd-4522
8. Rosuvastatin (inn)
9. Chembl1496
10. Chebi:38545
11. 413kh5zj73
12. Zd 4522
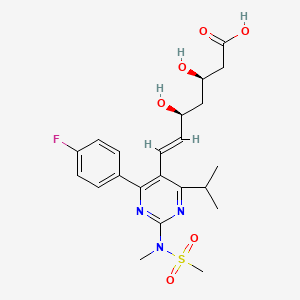
13. (3r,5s,6e)-7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(ethyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-6-heptenoic Acid
14. (3r,5s,6e)-7-[4-(4-fluorophenyl)-2-(n-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic Acid
15. (3r,5s,6e)-7-{4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl}-3,5-dihydroxyhept-6-enoic Acid
16. (3r,5s,e)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(n-methylmethylsulfonamido)pyrimidin-5-yl)-3,5-dihydroxyhept-6-enoic Acid
17. (e)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl](3r,5s)-3,5-dihydroxyhept-6-enoic Acid
18. 6-heptenoic Acid, 7-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]-3,5-dihydroxy-, (3r,5s,6e)-
19. Creston (tn)
20. Rosuvastatin [inn:ban]
21. Unii-413kh5zj73
22. Hsdb 7317
23. (3r,5s,6e)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(methyl(methylsulfonyl)amino)pyrimidin-5-yl)-3,5-dihydroxyhept-6-enoic Acid
24. 6-heptenoic Acid, 7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(methyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-, (3r,5s,6e)-
25. Spectrum5_001695
26. Rosuvastatin [mi]
27. Rosuvastatin [hsdb]
28. Schembl2520
29. Rosuvastatin [vandf]
30. Bspbio_003429
31. 6-heptenoic Acid, 7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(ethyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-, (3r,5s,6e)
32. Rosuvastatin [who-dd]
33. Schembl154400
34. Spectrum1505213
35. Gtpl2954
36. Dtxsid8048492
37. Bdbm18372
38. Chebi:93454
39. Hms1922n09
40. Zinc1535101
41. Ccg-40119
42. Hy-17504a
43. Mfcd18783208
44. Akos000280777
45. Am84890
46. Db01098
47. Ncgc00178070-01
48. (3r,5s,6e)-7-{4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-(propan-2-yl)pyrimidin-5-yl}-3,5-dihydroxyhept-6-enoic Acid
49. Ac-30585
50. As-12247
51. Sbi-0206727.p001
52. S5072
53. A24862
54. D08492
55. Q415159
56. Brd-k82941592-001-01-3
57. Brd-k82941592-238-02-9
58. (3r,5s)-7-[4-(4-fluorophenyl)-2-(methyl-methylsulfonylamino)-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic Acid
59. (3r,5s)-trans-7-(4-(4-fluorophenyl)-6-isopropyl-2-(n-methylmethylsulfonamido)pyrimidin-5-yl)-3,5-dihydroxyhept-6-enoic Acid
60. (e)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl](3r,5s)-3,5-dihydroxyhept-6enoic Acid
61. (e)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl]-(3r,5s)-3,5-dihydroxyhept-6-enoic Acid
62. 7-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-(methyl-methylsulfonyl-amino)-pyrimidin-5-yl]-3,5-dihydroxy-hept-6-enoic Acid;rosuvastatin;rosuvastatin Acid
63. E-(7-{2-(n-methyl-n-methanesulfonylamino)-4-(4-fluorophenyl)-6-isopropyl-pyrimidin-5-yl}-(3r,5s)-3,5-dihydroxy-hept-6-enoic Acid]
64. E-7-[2-(n-methyl-n-methanesulfonylamino)-4-(4-fluorophenyl)-6-isopropyl-pyrimidin-5-yl]-(3r,5s)-3,5-dihydroxyhept-6-enoic Acid
65. Fbi
| Molecular Weight | 481.5 g/mol |
|---|---|
| Molecular Formula | C22H28FN3O6S |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 10 |
| Exact Mass | 481.16828496 g/mol |
| Monoisotopic Mass | 481.16828496 g/mol |
| Topological Polar Surface Area | 149 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 767 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Crestor |
| PubMed Health | Rosuvastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | CRESTOR (rosuvastatin calcium) is a synthetic lipid-lowering agent for oral administration. The chemical name for rosuvastatin calcium is bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhe... |
| Active Ingredient | Rosuvastatin calcium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Ipr |
| 2 of 2 | |
|---|---|
| Drug Name | Crestor |
| PubMed Health | Rosuvastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | CRESTOR (rosuvastatin calcium) is a synthetic lipid-lowering agent for oral administration. The chemical name for rosuvastatin calcium is bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhe... |
| Active Ingredient | Rosuvastatin calcium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Ipr |
Hydroxymethylglutaryl-CoA Reductase Inhibitors
National Library of Medicine's Medical Subject Headings. Rosuvastatin. Online file (MeSH, 2016). Available from, as of November 28, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Rosuvastatin is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of February 1, 2017: https://clinicaltrials.gov/ct2/results?term=ROSUVASTATIN&Search=Search
In individuals without clinically evident coronary heart disease but with an increased risk of cardiovascular disease based on age >/= 50 years old in men and >/= 60 years old in women, hsCRP >/= 2 mg/L, and the presence of at least one additional cardiovascular disease risk factor such as hypertension, low HDL-C, smoking, or a family history of premature coronary heart disease, Crestor is indicated to: reduce the risk of stroke, reduce the risk of myocardial infarction, reduce the risk of arterial revascularization procedures. /Included in US product label/
https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb0f3b5e-4bc6-41c9-66b9-6257e2513512
Crestor is indicated as adjunctive therapy to diet to slow the progression of atherosclerosis in adult patients as part of a treatment strategy to lower Total-C and LDL-C to target levels. /Included in US product label/
https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb0f3b5e-4bc6-41c9-66b9-6257e2513512
For more Therapeutic Uses (Complete) data for Rosuvastatin (11 total), please visit the HSDB record page.
Crestor is contraindicated for use in pregnant women since safety in pregnant women has not been established and there is no apparent benefit to therapy with Crestor during pregnancy. Because HMG-CoA reductase inhibitors decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, Crestor may cause fetal harm when administered to pregnant women. Crestor should be discontinued as soon as pregnancy is recognized.
NIH; DailyMed. Current Medication Information for Crestor (Rosuvastatin Calcium Tablet, Film-Coated) (Updated: May 2016). Available from, as of March 30, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb0f3b5e-4bc6-41c9-66b9-6257e2513512
Crestor should be prescribed with caution in patients with predisposing factors for myopathy (e.g., age >/= 65 years, inadequately treated hypothyroidism, renal impairment).
NIH; DailyMed. Current Medication Information for Crestor (Rosuvastatin Calcium Tablet, Film-Coated) (Updated: May 2016). Available from, as of March 30, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb0f3b5e-4bc6-41c9-66b9-6257e2513512
Myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported in patients receiving statins, including rosuvastatin. These adverse effects can occur at any dosage, but the risk is increased with the highest dosage of rosuvastatin (40 mg daily).
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1865
Immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, has been reported rarely in patients receiving statins. Immune-mediated necrotizing myopathy is characterized by proximal muscle weakness and elevated creatine kinase (CK, creatine phosphokinase, CPK) concentrations that persist despite discontinuance of statin therapy, necrotizing myopathy without substantial inflammation, and improvement following therapy with immunosuppressive agents.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1865
For more Drug Warnings (Complete) data for Rosuvastatin (22 total), please visit the HSDB record page.
The FDA monograph states that rosuvastatin is indicated as an adjunct to diet in the treatment of triglyceridemia, Primary Dysbetalipoproteinemia (Type III Hyperlipoproteinemia), and Homozygous Familial Hypercholesterolemia. The Health Canada monograph for rosuvastatin further specifies that rosuvastatin is indicated for the reduction of elevated total cholesterol (Total-C), LDL-C, ApoB, the Total-C/HDL-C ratio and triglycerides (TG) and for increasing HDL-C in hyperlipidemic and dyslipidemic conditions when response to diet and exercise alone has been inadequate. It is also indicated for the prevention of major cardiovascular events (including risk of myocardial infarction, nonfatal stroke, and coronary artery revascularization) in adult patients without documented history of cardiovascular or cerebrovascular events, but with at least two conventional risk factors for cardiovascular disease. Prescribing of statin medications is considered standard practice following any cardiovascular events and for people with a moderate to high risk of development of CVD. Statin-indicated conditions include diabetes mellitus, clinical atherosclerosis (including myocardial infarction, acute coronary syndromes, stable angina, documented coronary artery disease, stroke, trans ischemic attack (TIA), documented carotid disease, peripheral artery disease, and claudication), abdominal aortic aneurysm, chronic kidney disease, and severely elevated LDL-C levels.
Homozygous Familial Hypercholesterolaemia, Prevention of cardiovascular events, Primary combined (mixed) dyslipidaemia, Primary hypercholesterolaemia
Rosuvastatin is a synthetic, enantiomerically pure antilipemic agent. It is used to lower total cholesterol, low density lipoprotein-cholesterol (LDL-C), apolipoprotein B (apoB), non-high density lipoprotein-cholesterol (non-HDL-C), and trigleride (TG) plasma concentrations while increasing HDL-C concentrations. High LDL-C, low HDL-C and high TG concentrations in the plasma are associated with increased risk of atherosclerosis and cardiovascular disease. The total cholesterol to HDL-C ratio is a strong predictor of coronary artery disease and high ratios are associated with higher risk of disease. Increased levels of HDL-C are associated with lower cardiovascular risk. By decreasing LDL-C and TG and increasing HDL-C, rosuvastatin reduces the risk of cardiovascular morbidity and mortality. Elevated cholesterol levels, and in particular, elevated low-density lipoprotein (LDL) levels, are an important risk factor for the development of CVD. Use of statins to target and reduce LDL levels has been shown in a number of landmark studies to significantly reduce the risk of development of CVD and all-cause mortality. Statins are considered a cost-effective treatment option for CVD due to their evidence of reducing all-cause mortality including fatal and non-fatal CVD as well as the need for surgical revascularization or angioplasty following a heart attack. Evidence has shown that even for low-risk individuals (with <10% risk of a major vascular event occurring within 5 years) statins cause a 20%-22% relative reduction in major cardiovascular events (heart attack, stroke, coronary revascularization, and coronary death) for every 1 mmol/L reduction in LDL without any significant side effects or risks. **Skeletal Muscle Effects** Cases of myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with HMG-CoA reductase inhibitors, including rosuvastatin. These risks can occur at any dose level, but are increased at the highest dose (40 mg). Rosuvastatin should be prescribed with caution in patients with predisposing factors for myopathy (e.g., age 65 years, inadequately treated hypothyroidism, renal impairment). The risk of myopathy during treatment with rosuvastatin may be increased with concurrent administration of some other lipid-lowering therapies (such as [fenofibrate] or [niacin]), [gemfibrozil], [cyclosporine], [atazanavir]/[ritonavir], [lopinavir]/ritonavir, or [simeprevir]. Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors, including rosuvastatin, coadministered with [colchicine], and caution should therefore be exercised when prescribing these two medications together. Real-world data from observational studies has suggested that 10-15% of people taking statins may experience muscle aches at some point during treatment. **Liver Enzyme Abnormalities** Increases in serum transaminases have been reported with HMG-CoA reductase inhibitors, including rosuvastatin. In most cases, the elevations were transient and resolved or improved on continued therapy or after a brief interruption in therapy. There were two cases of jaundice, for which a relationship to rosuvastatin therapy could not be determined, which resolved after discontinuation of therapy. There were no cases of liver failure or irreversible liver disease in these trials. **Endocrine Effects** Increases in HbA1c and fasting serum glucose levels have been reported with HMG-CoA reductase inhibitors, including rosuvastatin calcium tablets. Based on clinical trial data with rosuvastatin, in some instances these increases may exceed the threshold for the diagnosis of diabetes mellitus. An in vitro study found that [atorvastatin], [pravastatin], [rosuvastatin], and [pitavastatin] exhibited a dose-dependent cytotoxic effect on human pancreas islet cells, with reductions in cell viability of 32, 41, 34 and 29%, respectively, versus control]. Moreover, insulin secretion rates were decreased by 34, 30, 27 and 19%, respectively, relative to control. HMG-CoA reductase inhibitors interfere with cholesterol synthesis and lower cholesterol levels and, as such, might theoretically blunt adrenal or gonadal steroid hormone production. Rosuvastatin demonstrated no effect upon nonstimulated cortisol levels and no effect on thyroid metabolism as assessed by TSH plasma concentration. In rosuvastatin treated patients, there was no impairment of adrenocortical reserve and no reduction in plasma cortisol concentrations. Clinical studies with other HMG-CoA reductase inhibitors have suggested that these agents do not reduce plasma testosterone concentration. The effects of HMG-CoA reductase inhibitors on male fertility have not been studied. The effects, if any, on the pituitarygonadal axis in premenopausal women are unknown. **Cardiovascular** Ubiquinone levels were not measured in rosuvastatin clinical trials, however significant decreases in circulating ubiquinone levels in patients treated with other statins have been observed. The clinical significance of a potential long-term statin-induced deficiency of ubiquinone has not been established. It has been reported that a decrease in myocardial ubiquinone levels could lead to impaired cardiac function in patients with borderline congestive heart failure. **Lipoprotein A** In some patients, the beneficial effect of lowered total cholesterol and LDL-C levels may be partly blunted by a concomitant increase in the Lipoprotein(a) [Lp(a)] concentrations. Present knowledge suggests the importance of high Lp(a) levels as an emerging risk factor for coronary heart disease. It is thus desirable to maintain and reinforce lifestyle changes in high-risk patients placed on rosuvastatin therapy. Further studies have demonstrated statins affect Lp(a) levels differently in patients with dyslipidemia depending on their apo(a) phenotype; statins increase Lp(a) levels exclusively in patients with the low molecular weight apo(a) phenotype.
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Compounds that inhibit HYDROXYMETHYLGLUTARYL COA REDUCTASES. They have been shown to directly lower CHOLESTEROL synthesis. (See all compounds classified as Hydroxymethylglutaryl-CoA Reductase Inhibitors.)
Anticholesteremic Agents
Substances used to lower plasma cholesterol levels. (See all compounds classified as Anticholesteremic Agents.)
C10AA07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C10 - Lipid modifying agents
C10A - Lipid modifying agents, plain
C10AA - Hmg coa reductase inhibitors
C10AA07 - Rosuvastatin
Absorption
In a study of healthy white male volunteers, the absolute oral bioavailability of rosuvastatin was found to be approximately 20% while absorption was estimated to be 50%, which is consistent with a substantial first-pass effect after oral dosing. Another study in healthy volunteers found that the peak plasma concentration (Cmax) of rosuvastatin was 6.06ng/mL and was reached at a median of 5 hours following oral dosing. Both Cmax and AUC increased in approximate proportion to dose. Neither food nor evening versus morning administration was shown to have an effect on the AUC of rosuvastatin. Many statins are known to interact with hepatic uptake transporters and thus reach high concentrations at their site of action in the liver. Breast Cancer Resistance Protein (BCRP) is a membrane-bound protein that plays an important role in the absorption of rosuvastatin, particularly as CYP3A4 has minimal involvement in its metabolism. Evidence from pharmacogenetic studies of c.421C>A single nucleotide polymorphisms (SNPs) in the gene for BCRP has demonstrated that individuals with the 421AA genotype have reduced functional activity and 2.4-fold higher AUC and Cmax values for rosuvastatin compared to study individuals with the control 421CC genotype. This has important implications for the variation in response to the drug in terms of efficacy and toxicity, particularly as the BCRP c.421C>A polymorphism occurs more frequently in Asian populations than in Caucasians. Other statin drugs impacted by this polymorphism include [fluvastatin] and [atorvastatin]. Genetic differences in the OATP1B1 (organic-anion-transporting polypeptide 1B1) hepatic transporter have also been shown to impact rosuvastatin pharmacokinetics. Evidence from pharmacogenetic studies of the c.521T>C SNP showed that rosuvastatin AUC was increased 1.62-fold for individuals homozygous for 521CC compared to homozygous 521TT individuals. Other statin drugs impacted by this polymorphism include [simvastatin], [pitavastatin], [atorvastatin], and [pravastatin]. For patients known to have the above-mentioned c.421AA BCRP or c.521CC OATP1B1 genotypes, a maximum daily dose of 20mg of rosuvastatin is recommended to avoid adverse effects from the increased exposure to the drug, such as muscle pain and risk of rhabdomyolysis.
Route of Elimination
Rosuvastatin is not extensively metabolized; approximately 10% of a radiolabeled dose is recovered as metabolite. Following oral administration, rosuvastatin and its metabolites are primarily excreted in the feces (90%). After an intravenous dose, approximately 28% of total body clearance was via the renal route, and 72% by the hepatic route. A study in healthy adult male volunteers found that approximately 90% of the rosuvastatin dose was recovered in feces within 72 hours after dose, while the remaining 10% was recovered in urine. The drug was completely excreted from the body after 10 days of dosing. They also found that approximately 76.8% of the excreted dose was unchanged from the parent compound, with the remaining dose recovered as the metabolites n-desmethyl rosuvastatin and rosuvastatin-5S-lactone. Renal tubular secretion is responsible for >90% of total renal clearance, and is believed to be mediated primarily by the uptake transporter OAT3 (Organic anion transporter 1), while OAT1 had minimal involvement.
Volume of Distribution
Rosuvastatin undergoes first-pass extraction in the liver, which is the primary site of cholesterol synthesis and LDL-C clearance. The mean volume of distribution at steady-state of rosuvastatin is approximately 134 litres.
In clinical pharmacology studies in man, peak plasma concentrations of rosuvastatin were reached 3 to 5 hours following oral dosing. Both Cmax and AUC increased in approximate proportion to Crestor dose. The absolute bioavailability of rosuvastatin is approximately 20%. Administration of Crestor with food did not affect the AUC of rosuvastatin. The AUC of rosuvastatin does not differ following evening or morning drug administration.
NIH; DailyMed. Current Medication Information for Crestor (Rosuvastatin Calcium Tablet, Film-Coated) (Updated: May 2016). Available from, as of March 30, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb0f3b5e-4bc6-41c9-66b9-6257e2513512
Mean volume of distribution at steady-state of rosuvastatin is approximately 134 liters. Rosuvastatin is 88% bound to plasma proteins, mostly albumin. This binding is reversible and independent of plasma concentrations.
NIH; DailyMed. Current Medication Information for Crestor (Rosuvastatin Calcium Tablet, Film-Coated) (Updated: May 2016). Available from, as of March 30, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb0f3b5e-4bc6-41c9-66b9-6257e2513512
Following oral administration, rosuvastatin and its metabolites are primarily excreted in the feces (90%). ... After an intravenous dose, approximately 28% of total body clearance was via the renal route, and 72% by the hepatic route.
NIH; DailyMed. Current Medication Information for Crestor (Rosuvastatin Calcium Tablet, Film-Coated) (Updated: May 2016). Available from, as of March 30, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb0f3b5e-4bc6-41c9-66b9-6257e2513512
/MILK/ Limited data indicate that Crestor is present in human milk.
NIH; DailyMed. Current Medication Information for Crestor (Rosuvastatin Calcium Tablet, Film-Coated) (Updated: May 2016). Available from, as of March 30, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb0f3b5e-4bc6-41c9-66b9-6257e2513512
For more Absorption, Distribution and Excretion (Complete) data for Rosuvastatin (7 total), please visit the HSDB record page.
Rosuvastatin is not extensively metabolized, as demonstrated by the small amount of radiolabeled dose that is recovered as a metabolite (~10%). Cytochrome P450 (CYP) 2C9 is primarily responsible for the formation of rosuvastatin's major metabolite, N-desmethylrosuvastatin, which has approximately 20-50% of the pharmacological activity of its parent compound in vitro. However, this metabolic pathway isn't deemed to be clinically significant as there were no observable effects found on rosuvastatin pharmacokinetics when rosuvastatin was coadministered with fluconazole, a potent CYP2C9 inhibitor. In vitro and in vivo data indicate that rosuvastatin has no clinically significant cytochrome P450 interactions (as substrate, inhibitor or inducer). Consequently, there is little potential for drug-drug interactions upon coadministration with agents that are metabolized by cytochrome P450.
Rosuvastatin is not extensively metabolized; approximately 10% of a radiolabeled dose is recovered as metabolite. The major metabolite is N-desmethyl rosuvastatin, which is formed principally by cytochrome P450 \ 2C9, and in vitro studies have demonstrated that N-desmethyl rosuvastatin has approximately one-sixth to one-half the HMG-CoA reductase inhibitory activity of the parent compound. Overall, greater than 90% of active plasma HMG-CoA reductase inhibitory activity is accounted for by the parent compound.
NIH; DailyMed. Current Medication Information for Crestor (Rosuvastatin Calcium Tablet, Film-Coated) (Updated: May 2016). Available from, as of March 30, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb0f3b5e-4bc6-41c9-66b9-6257e2513512
The elimination half-life (t) of rosuvastatin is approximately 19 hours and does not increase with increasing doses.
The elimination half-life of rosuvastatin is approximately 19 hours.
NIH; DailyMed. Current Medication Information for Crestor (Rosuvastatin Calcium Tablet, Film-Coated) (Updated: May 2016). Available from, as of March 30, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb0f3b5e-4bc6-41c9-66b9-6257e2513512
Rosuvastatin is a statin medication and a competitive inhibitor of the enzyme HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase, which catalyzes the conversion of HMG-CoA to mevalonate, an early rate-limiting step in cholesterol biosynthesis. Rosuvastatin acts primarily in the liver, where decreased hepatic cholesterol concentrations stimulate the upregulation of hepatic low density lipoprotein (LDL) receptors which increases hepatic uptake of LDL. Rosuvastatin also inhibits hepatic synthesis of very low density lipoprotein (VLDL). The overall effect is a decrease in plasma LDL and VLDL. In vitro and in vivo animal studies also demonstrate that rosuvastatin exerts vasculoprotective effects independent of its lipid-lowering properties, also known as the pleiotropic effects of statins. This includes improvement in endothelial function, enhanced stability of atherosclerotic plaques, reduced oxidative stress and inflammation, and inhibition of the thrombogenic response. Statins have also been found to bind allosterically to 2 integrin function-associated antigen-1 (LFA-1), which plays an important role in leukocyte trafficking and in T cell activation. Rosuvastatin exerts an anti-inflammatory effect on rat mesenteric microvascular endothelium by attenuating leukocyte rolling, adherence and transmigration. The drug also modulates nitric oxide synthase (NOS) expression and reduces ischemic-reperfusion injuries in rat hearts. Rosuvastatin increases the bioavailability of nitric oxide by upregulating NOS and by increasing the stability of NOS through post-transcriptional polyadenylation. It is unclear as to how rosuvastatin brings about these effects though they may be due to decreased concentrations of mevalonic acid.
Crestor is a selective and competitive inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts 3-hydroxy-3-methylglutaryl coenzyme A to mevalonate, a precursor of cholesterol. In vivo studies in animals, and in vitro studies in cultured animal and human cells have shown rosuvastatin to have a high uptake into, and selectivity for, action in the liver, the target organ for cholesterol lowering. In in vivo and in vitro studies, rosuvastatin produces its lipid-modifying effects in two ways. First, it increases the number of hepatic LDL receptors on the cell-surface to enhance uptake and catabolism of LDL. Second, rosuvastatin inhibits hepatic synthesis of VLDL, which reduces the total number of VLDL and LDL particles.
NIH; DailyMed. Current Medication Information for Crestor (Rosuvastatin Calcium Tablet, Film-Coated) (Updated: May 2016). Available from, as of March 30, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb0f3b5e-4bc6-41c9-66b9-6257e2513512

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Regulatory Info :
Registration Country : Netherlands
Brand Name :
Dosage Form : Film Coated Tablet
Dosage Strength : 5MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Netherlands
 Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Packaging :
Regulatory Info :
Dosage : Film Coated Tablet
Dosage Strength : 5MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Netherlands
 Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Regulatory Info :
Registration Country : Netherlands
Brand Name :
Dosage Form : Film Coated Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Netherlands
 Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Packaging :
Regulatory Info :
Dosage : Film Coated Tablet
Dosage Strength : 10MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Netherlands
 Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Regulatory Info :
Registration Country : Netherlands
Brand Name :
Dosage Form : Film Coated Tablet
Dosage Strength : 20MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Netherlands
 Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Packaging :
Regulatory Info :
Dosage : Film Coated Tablet
Dosage Strength : 20MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Netherlands
 Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Regulatory Info :
Registration Country : Netherlands
Brand Name :
Dosage Form : Film Coated Tablet
Dosage Strength : 40MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Netherlands
 Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Packaging :
Regulatory Info :
Dosage : Film Coated Tablet
Dosage Strength : 40MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Netherlands
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Regulatory Info :
Registration Country : Hungary
Brand Name :
Dosage Form : Film Coated Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Hungary
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Packaging :
Regulatory Info :
Dosage : Film Coated Tablet
Dosage Strength : 10MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Hungary
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Regulatory Info :
Registration Country : Hungary
Brand Name :
Dosage Form : Film Coated Tablet
Dosage Strength : 20MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Hungary
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Packaging :
Regulatory Info :
Dosage : Film Coated Tablet
Dosage Strength : 20MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Hungary
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Regulatory Info :
Registration Country : Hungary
Brand Name :
Dosage Form : Film Coated Tablet
Dosage Strength : 40MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Hungary
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Packaging :
Regulatory Info :
Dosage : Film Coated Tablet
Dosage Strength : 40MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Hungary
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Regulatory Info :
Registration Country : Hungary
Brand Name :
Dosage Form : Capsule
Dosage Strength : 40MG; 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Hungary
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Packaging :
Regulatory Info :
Dosage : Capsule
Dosage Strength : 40MG; 10MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Hungary
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Regulatory Info :
Registration Country : Hungary
Brand Name :
Dosage Form : Capsule
Dosage Strength : 10MG; 5MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Hungary
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Packaging :
Regulatory Info :
Dosage : Capsule
Dosage Strength : 10MG; 5MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Hungary
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Regulatory Info :
Registration Country : Hungary
Brand Name :
Dosage Form : Capsule
Dosage Strength : 10MG; 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Hungary
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Packaging :
Regulatory Info :
Dosage : Capsule
Dosage Strength : 10MG; 10MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Hungary
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
19
PharmaCompass offers a list of Rosuvastatin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Rosuvastatin manufacturer or Rosuvastatin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Rosuvastatin manufacturer or Rosuvastatin supplier.
PharmaCompass also assists you with knowing the Rosuvastatin API Price utilized in the formulation of products. Rosuvastatin API Price is not always fixed or binding as the Rosuvastatin Price is obtained through a variety of data sources. The Rosuvastatin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Crestor manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Crestor, including repackagers and relabelers. The FDA regulates Crestor manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Crestor API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Crestor manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Crestor supplier is an individual or a company that provides Crestor active pharmaceutical ingredient (API) or Crestor finished formulations upon request. The Crestor suppliers may include Crestor API manufacturers, exporters, distributors and traders.
click here to find a list of Crestor suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Crestor DMF (Drug Master File) is a document detailing the whole manufacturing process of Crestor active pharmaceutical ingredient (API) in detail. Different forms of Crestor DMFs exist exist since differing nations have different regulations, such as Crestor USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Crestor DMF submitted to regulatory agencies in the US is known as a USDMF. Crestor USDMF includes data on Crestor's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Crestor USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Crestor suppliers with USDMF on PharmaCompass.
Crestor Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Crestor GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Crestor GMP manufacturer or Crestor GMP API supplier for your needs.
A Crestor CoA (Certificate of Analysis) is a formal document that attests to Crestor's compliance with Crestor specifications and serves as a tool for batch-level quality control.
Crestor CoA mostly includes findings from lab analyses of a specific batch. For each Crestor CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Crestor may be tested according to a variety of international standards, such as European Pharmacopoeia (Crestor EP), Crestor JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Crestor USP).
