
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
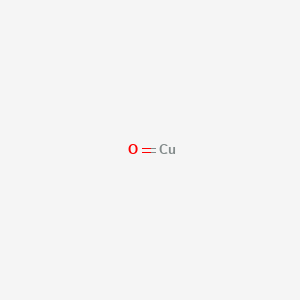

1. Copper Dioxide (cuo2)
2. Copper Oxide (cuo)
3. Cupric Oxide
1. Oxocopper
2. Copper Oxide (cuo)
3. Copper Monoxide
4. Banacobru Ol
5. Chrome Brown
6. Copper Brown
7. Copper Monooxide
8. Copper(2+) Oxide
9. C.i. Pigment Black 15
10. Copper (ii) Oxide
11. Cu-o Linkage
12. C.i. 77403
13. Mfcd00010979
14. Cuo
15. Paramelaconite
16. Copacaps
17. Copporal
18. Natural Tenorite
19. Wolmanac Concentrate
20. Boliden Salt K-33
21. Caswell No. 265
22. Ci Pigment Black 15
23. Boliden-cca Wood Preservative
24. Cca Type C Wood Preservative
25. Hsdb 266
26. Osmose K-33 Wood Preservative
27. Osmose P-50 Wood Preservative
28. Osmose K-33-a Wood Preservative
29. Osmose K-33-c Wood Preservative
30. Einecs 215-269-1
31. Nsc 83537
32. Epa Pesticide Chemical Code 042401
33. Unii-v1xjq704r4
34. Ci 77403
35. Copper-oxygen
36. Farboil Super Tropical Anti-fouling 1260
37. Copper(ii)oxide
38. Copper Ii Oxide
39. Copper Oxide Ink
40. Copper Oxide, Cuo
41. Copper Oxide Powder
42. Copper-(ii) Oxide
43. Copper Oxide Dispersion
44. Copper Oxide Nanopowder
45. Cupric Oxide Nanopowder
46. Copper Oxide Nano-chains
47. Copper(ii) Oxide, Cp
48. Copper Oxide Nanoparticles
49. Copper(ii) Oxide, Powder
50. Ec 215-269-1
51. Copper(ii) Oxide, Puratronic?
52. Copper Oxide Powder, 99+% Nano
53. Nsc83537
54. Copper(ii) Oxide, Lr, >=97%
55. Copper Oxide Nanoparticles Dispersion
56. Akos015950660
57. Copper(ii) Oxide (99.995%-cu)
58. Copper Oxide Nanoparticles / Nanopowder
59. Copper(ii) Oxide, Acs Reagent, >=99.0%
60. Copper(ii) Oxide, Powder, <10 Mum, 98%
61. Cs-0016015
62. Ft-0624050
63. Chromium Silicide (crsi2) Sputtering Targets
64. Copper(ii) Oxide, >=99.0% (rt), Granular
65. Copper(ii) Oxide, 99.999% Trace Metals Basis
66. Copper(ii) Oxide, P.a., Acs Reagent, 99.0%
67. J-520121
68. Q27458610
69. Copper(ii) Oxide, Powder, 99.99% Trace Metals Basis
70. Copper(ii) Oxide, Powder, 99.995% Trace Metals Basis
71. Copper(ii) Oxide, Nanopowder, <50 Nm Particle Size (tem)
72. Copper(ii) Oxide, Puriss. P.a., >=99.0% (rt), Powder
73. Copper(ii) Oxide, Nanotubes, Diam. X L 10-12 Nm X 75-100 Nm
74. Copper(ii) Oxide, Needles, Mixture Of Cuo And Cu2o, Acs Reagent
75. Pedot Pss Poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate )
76. Copper(ii) Oxide On Alumina, 14-20 Mesh, Extent Of Labeling: 13 Wt. % Loading
| Molecular Weight | 79.55 g/mol |
|---|---|
| Molecular Formula | CuO |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 78.924512 g/mol |
| Monoisotopic Mass | 78.924512 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Trace Elements
A group of chemical elements that are needed in minute quantities for the proper growth, development, and physiology of an organism. (From McGraw-Hill Dictionary of Scientific and Technical Terms, 4th ed) (See all compounds classified as Trace Elements.)
The pulmonary uptake of copper oxide /occurred/ in rats exposed to aerosols containing 50-80 mg/cu m. Animals were exposed for 15, 30, 45, or 60 minutes and killed immediately. Another group was exposed for 180 minutes and killed at 0, 3, 6, 12, 18, or 24 hours after exposure. Electron microscopic histologic examination showed that absorption of copper had occurred in animals exposed for 180 minutes. Copper oxide particles penetrated the epithelial cells of alveoli and were found in plasma 6 hours after exposure began. Copper oxide was also observed in the proximal convoluted tubules of the kidney. /Copper oxide/
USEPA; Drinking Water Criteria Document for Copper (Final Draft) p.III-3 (1985) EPA-600/X-84-190-1
VET: COPPER SOURCE. 80% COPPER CONTENT. LOW ABSORPTION RATE & HIGH FECAL EXCRETION RATE IN CATTLE & SWINE FEEDING TRIALS. IN GENERAL MONOGASTRIC ANIMALS UTILIZE /CUPRIC OXIDE/ BETTER THAN RUMINANTS. POULTRY UTILIZATION IS SOMEWHERE BETWEEN THE TWO.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 135
Cheviot ewes (mean live weight 50 kg) were given single doses of 0, 2.5, 5, 10, or 20 g cupric oxide particles in gelatin capsules while receiving a diet of marginal copper content based on pelleted oats. After 65 days, liver copper concentrations had increased curvilinearly in relation to dose and all ewes given 10 or 20 g cupric oxide particles showed increases of at least 13.4 mmol/kg dry matter (850 ppm). Liver copper concentrations had generally declined after 85 days but biochemical and histological evidence of copper toxicity was recorded in one ewe which had received 20 g cupric oxide particles. Despite marked variations between individual sheep, a dose of 0.1 g/kg liveweight (5 g) was considered to be safe and did not induce clinical copper toxicity in five sheep of the susceptible North Ronaldsay breed given the same basal diet.
PMID:3589168 Suttle NF; Res Vet Sci 42 (2): 219-23 (1987)
Crossbred steers, mean initial live weight 220 kg, were given a diet of barley and hay ad libitum. Each animal received a single oral does of 0, 5, 10, 20, or 40 g cupric oxide particles. A dose of 5 g cupric oxide particles increased liver copper stores for about 240 days and higher doses increased liver stores for longer but 40 g was no more effective than 20 g (85 mg/kg live weight). Variation among individuals was marked but the highest liver copper concentration recorded (7.59 mmol/kg dry matter) produced no biochemical evidence of copper toxicity. Cupric oxide particles were separated into three fractions, clumps, short rods and long; and 5 mg/kg live weight of each fraction given to steers of 173 kg mean live weight. The form of the particles did not affect either their retention in the alimentary tract or the accumulation of copper in the liver.
PMID:3589169 Suttle NF; Res Vet Sci 42 (2): 224-7 (1987)
For more Absorption, Distribution and Excretion (Complete) data for COPPER(II) OXIDE (6 total), please visit the HSDB record page.
Related Excipient Companies

Excipients by Applications
Market Place

ABOUT THIS PAGE
23
PharmaCompass offers a list of Copper Oxide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Copper Oxide manufacturer or Copper Oxide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Copper Oxide manufacturer or Copper Oxide supplier.
PharmaCompass also assists you with knowing the Copper Oxide API Price utilized in the formulation of products. Copper Oxide API Price is not always fixed or binding as the Copper Oxide Price is obtained through a variety of data sources. The Copper Oxide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Cupric Oxide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Cupric Oxide, including repackagers and relabelers. The FDA regulates Cupric Oxide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Cupric Oxide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Cupric Oxide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Cupric Oxide supplier is an individual or a company that provides Cupric Oxide active pharmaceutical ingredient (API) or Cupric Oxide finished formulations upon request. The Cupric Oxide suppliers may include Cupric Oxide API manufacturers, exporters, distributors and traders.
click here to find a list of Cupric Oxide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Cupric Oxide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Cupric Oxide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Cupric Oxide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Cupric Oxide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Cupric Oxide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Cupric Oxide suppliers with NDC on PharmaCompass.
Cupric Oxide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Cupric Oxide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Cupric Oxide GMP manufacturer or Cupric Oxide GMP API supplier for your needs.
A Cupric Oxide CoA (Certificate of Analysis) is a formal document that attests to Cupric Oxide's compliance with Cupric Oxide specifications and serves as a tool for batch-level quality control.
Cupric Oxide CoA mostly includes findings from lab analyses of a specific batch. For each Cupric Oxide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Cupric Oxide may be tested according to a variety of international standards, such as European Pharmacopoeia (Cupric Oxide EP), Cupric Oxide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Cupric Oxide USP).
