
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
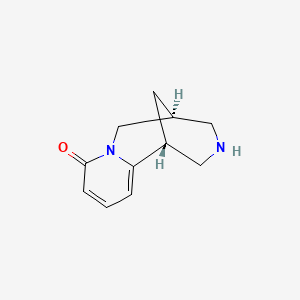

1. 3-hydroxy-11-norcytisine
2. Citizine
3. Cystisin
4. Cytisine Dihydrochloride, Trihydrate
5. Cytisine Hydrochloride
6. Cytisine Hydrochloride, Hydrate
7. Cytisine Tetrahydrochloride, Trihydrate
8. Cytiton
9. Tabex
10. Tsitizin
1. 485-35-8
2. Sophorine
3. Baptitoxine
4. Cytiton
5. Baptitoxin
6. Cytisinicline
7. Laburnin
8. Tabex
9. Ulexine
10. Ulexin
11. (-)-cytisine
12. Citizin
13. Cytitone
14. Tsitizin
15. Cystisine
16. Cytizin
17. Tabax
18. Baphitoxine
19. (1r,5s)-3,4,5,6-tetrahydro-1h-1,5-methanopyrido[1,2-a][1,5]diazocin-8(2h)-one
20. (1r,5s)-1,2,3,4,5,6-hexahydro-8h-1,5-methanopyrido[1,2-a][1,5]diazocin-8-one
21. 6039 Sopharma
22. Cytisin
23. Cytisine (-)
24. Cytisinicline [usan]
25. Nsc-407282
26. Chebi:4055
27. Chembl497939
28. 53s5u404nu
29. (1r,9s)-7,11-diazatricyclo[7.3.1.0^{2,7}]trideca-2,4-dien-6-one
30. (1r,9s)-7,11-diazatricyclo[7.3.1.02,7]trideca-2,4-dien-6-one
31. Nsc 407282
32. Brn 0083882
33. 1,5-methano-8h-pyrido(1,2-a)(1,5)diazocin-8-one, 1,2,3,4,5,6-hexahydro-, (1r,5s)-
34. 1,5-methano-8h-pyrido[1,2-a][1,5]diazocin-8-one, 1,2,3,4,5,6-hexahydro-, (1r,5s)-
35. Smr001233264
36. Hsdb 3560
37. Mls003171607
38. Einecs 207-616-0
39. Unii-53s5u404nu
40. Mfcd00136048
41. Nsc407282
42. 1,2,3,4,5,6-hexahydro-1,5-methano-8h-pyrido(1,2-a)(1,5)diazocin-8-one
43. Cytisine- Bio-x
44. C5e
45. Prestwick_140
46. Cytisine ((+)-)
47. Tocris-1390
48. Cytisine [hsdb]
49. Cytisine [mi]
50. Prestwick3_000624
51. Cytisine [mart.]
52. Cytisinicline [inn]
53. (1r,5s)-1,2,3,4,5,6-hexahydro-1,5-methano-8h-pyrido[1,2-a][1,5]diazocin-8-one
54. Bspbio_000588
55. Cytisine, >=99%, Powder
56. 5-24-02-00535 (beilstein Handbook Reference)
57. Mls002153916
58. Mls002222174
59. Schembl161398
60. Cytisinicline [who-dd]
61. Bpbio1_000648
62. Gtpl5347
63. (1r,9r)-7,11-diazatricyclo[7.3.1.0~2,7~]trideca-2,4-dien-6-one
64. (1s,9r)-7,11-diazatricyclo[7.3.1.0~2,7~]trideca-2,4-dien-6-one
65. 1,2,3,4,5,6-hexahydro-1,5-methano-pyrido[1,2-a][1,5]diazocin-8-one
66. Dtxsid00883395
67. 1,2,3,4,5,6-hexahydro-1,5-methano-pyrido[1,2-a][1,5]diazocin-8-one (cytisine)
68. Cytisine1,2,3,4,5,6-hexahydro-1,5-methano-pyrido[1,2-a][1,5]diazocin-8-one
69. Hms2096n10
70. Hms2235l06
71. Hms3267d20
72. Hms3414n03
73. Hms3678l21
74. Hms3884l11
75. Hy-n0175
76. Tnp00030
77. Zinc1599730
78. Bdbm50143282
79. Pdsp1_000461
80. S2287
81. Wln: T C666 A Gvn Lm&ttj
82. Akos000276818
83. Akos015833102
84. Ccg-208621
85. Db09028
86. (1r,5s)-1,2,3,4,5,6-hexahydro-1,5-methano-8h-pyrido[1,2a][1,5]diazocin-8-one
87. Ncgc00016463-01
88. Ncgc00016463-02
89. Ncgc00016463-03
90. Ncgc00016463-04
91. Ncgc00017171-01
92. Ncgc00025138-01
93. Ncgc00025138-02
94. Ncgc00179513-01
95. Ac-34317
96. As-19539
97. Bc164343
98. Cas-485-35-8
99. Cs-0007888
100. Sw219952-1
101. Cytisine, >=99.0% (hplc), >=99%
102. 485c358
103. Q417343
104. Brd-k74186897-001-02-5
105. Brd-k74186897-001-04-1
106. Brd-k74186897-001-13-2
107. F9994-5373
108. Z2582785591
109. 1,2-a][1,5]diazocin-8-one, 1,2,3,4,5,6-hexahydro-
110. 1,2-a][1,5]diazocin-8-one, 1,2,3,4,5,6-hexahydro-, (1r)-
111. (1r,9s)-7,11-diazatricyclo[7.3.1.0~2,7~]trideca-2,4-dien-6-one
112. 1,2-a][1,5]diazocin-8-one, 1,2,3,4,5,6-hexahydro-, (1r-cis)-
113. (1r,5s)-1,2,3,4,5,6-hexahydro-1,5-methano-pyrido[1,2-a][1,5]diazocin-8-one
114. (1r,5s)-1,2,3,4,5,6-hexahydro-1,5-methano-8h-pyrido-(1,2-a)(1,5)diazocin-8-one
115. (1r,5s)-1,2,3,4,5,6-hexahydro-8h-1,5-methanopyrido(1,2-a)(1,5)diazocin-8-one (cytisine)
116. 115051-74-6
| Molecular Weight | 190.24 g/mol |
|---|---|
| Molecular Formula | C11H14N2O |
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 190.110613074 g/mol |
| Monoisotopic Mass | 190.110613074 g/mol |
| Topological Polar Surface Area | 32.3 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 332 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Nicotine/antagonists and inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
/Cytisine is indicated for/ treatment of chronic nicotinism. It is particularly appropriate for treatment of risk groups of smokers with health problems on the part of the cardiovascular and respiratory systems, as well as smokers professionally subjected to tension and stress that are predisposed to seek a "false comfort" in nicotine or other drugs causing dependence. /NOT included in US product label/
Sopharma AD; Patient Information for Tabex tablets (2006).
/Investigators/ conducted a single-center, randomized, double-blind, placebo-controlled trial. Participants were randomly assigned to receive cytisine or matching placebo for 25 days; participants in both groups received a minimal amount of counseling during the study. The primary outcome measure was sustained, biochemically verified smoking abstinence for 12 months after the end of treatment. Of 1542 adult smokers screened, 740 were enrolled and 370 were randomly assigned to each study group. The rate of sustained 12-month abstinence was 8.4% (31 participants) in the cytisine group as compared with 2.4% (9 participants) in the placebo group (difference, 6.0 percentage points; 95% confidence interval (CI), 2.7 to 9.2; P=0.001). The 7-day point prevalence for abstinence at the 12-month follow-up was 13.2% in the cytisine group versus 7.3% in the placebo group (P=0.01). Gastrointestinal adverse events were reported more frequently in the cytisine group (difference, 5.7 percentage points; 95% CI, 1.2 to 10.2). ...
PMID:21991893 West R et al; N Engl J Med 365: 1193-1200 (2011)
Cytisine, a natural plant alkaloid, has been marketed in Central and Eastern Europe for over 40 years for the clinical management of smoking cessation. Despite the fact that cytisine has been used by millions of smokers, its characteristics have not been reviewed in scientific literature in English, and presently existing clinical studies on its effectiveness and safety are insufficient to warrant licensing by modern standards. Understanding of the mechanism of cytisine action as a smoking cessation aid provides a necessary basis for conducting clinical trials to confirm its efficacy as an optimal antismoking therapy. ... /Investigators/ present a review of current knowledge about the pharmacokinetics, pharmacodynamics, toxicity, therapeutic efficacy and safety of cytisine, and about its derivatives that are under development. Recent pharmacological research has elucidated that the drug is a low efficacy partial agonist of alpha4beta2 nicotinic acetylcholine receptors, which are believed to be central to the effect of nicotine (NIC) on the reward pathway. The drug reduces the effects of NIC on dopamine release in the mesolimbic system when given alone, while simultaneously attenuating NIC withdrawal symptoms that accompany cessation attempts. Clinical studies on cytisine as a smoking cessation aid have demonstrated that the drug is effective and safe. /The/ recent uncontrolled trial has shown that a 12-month carbon monoxide-verified continuous abstinence rate following a standard course of treatment with cytisine with minimal behavioral support is similar (13.8%; N = 436) to that observed following treatment with NIC replacement therapy. Since cytisine exhibits a desirable pharmacological profile which makes it an attractive smoking cessation drug, it should be advanced to randomized clinical trials. However, more detailed preclinical studies on its pharmacokinetics and safety profile are required.
PMID:17220536 Tutka P, Zatonski W; Pharmacol Rep 58 (6): 777-98 (2006)
/Contraindications for cytisine include:/ Advanced atherosclerosis, some forms of schizophrenia, pheochromocytome, conditions connected with severe impairment of the cardiovascular system and malignant hypertension.
Sopharma AD; Patient Information for Tabex tablets (2006).
The following adverse effects are rather often observed at the beginning of /Cytisine/ treatment: changes in both taste and appetite, dryness in the mouth, headache, irritability, nausea, constipation, tachycardia, light elevation of the arterial pressure.
Sopharma AD; Patient Information for Tabex tablets (2006).
The drug should be administered carefully to patients with exacerbated peptic ulcer. After completing the treatment course, the patients should refrain from smoking...
Sopharma AD; Patient Information for Tabex tablets (2006).
The physicians should warn the patients that the simultaneous administration of the drug and smoking could lead to aggravated adverse effects of nicotine (nicotine intoxication). The drug should be used in all cases when the patient has a honest and firm intention to give up smoking.
Sopharma AD; Patient Information for Tabex tablets (2006).
For more Drug Warnings (Complete) data for CYTISINE (8 total), please visit the HSDB record page.
Indicated for use in smoking cessation.
Various models have been run where the affinity of nAChR agonists to the receptor subtype are tested to help identify the molecules, groups and steric conformation that are vital to greater affinity. By using a nAChR muscle receptor subtype (1)21 model the following results were obtained: anatoxin > epibatidine > acetylcholine > DMPP >> CYTISINE > pyrantel > nicotine > coniine > tubocurare > lobeline, where anatoxin had the highest activity efficacy and tubocurare the lowest. Acetylcholine on the other hand induced a much longer opening time of the receptor though anatoxin is more potent. The results suggest that anatoxin derivatives would be helpful in understanding structure-activity relationships (SAR) for muscle nAChRs (Cooper et al., 1996).
N - Nervous system
N07 - Other nervous system drugs
N07B - Drugs used in addictive disorders
N07BA - Drugs used in nicotine dependence
N07BA04 - Cytisinicline
Volume of Distribution
Oral: 6.2 l/kg Oral (5 mg/kg; tested in rabbits).
Clearance
Renal: 43 mL/min.
The pharmacokinetic behavior of cytisine was studied in mice by means of tritiated cytisine after intravenous and oral administration of a sublethal dose of 2 mg/kg. After oral administration the maximum blood level is reached after 2 hr. The absorption rate is approximately 42%. From the blood level after intravenous administration a half-life of 200 min was calculated. Within 24 hr after intravenous administration 32% and after oral administration 18% of the administered radioactivity was excreted into urine. Following intravenous administration 3% of the dose was found in the feces within 6 hr. Among the examined organs and tissues the highest concentrations were reached in the liver, adrenals and kidneys. In the bile the highest concentration after intravenous administration was 200 times that in the blood.
PMID:6933924 Klocking HP et al; Arch Toxicol Suppl 4: 312-4 (1980)
Experimental study of pharmacokinetics of transdermal system with cytisine in rabbits showed a possibility of controlled intake of the drug over a 4-day period. The two stages of attaining the stationary levels of cytisin concentrations are revealed. The first stage lasted during first 24 hr and the second stage during succeeding 3 days. Using the data on intravenous cytisine injection we found that the stationary concentrations and the rate of cytisine intake in the first stage is twice as large as in the second stage. Thus cytisine can be classed as a short-living drug.
PMID:10650531 Sariev AK et al; Eksp Klin Farmakol 62 (6): 59-61 (1999)
4.8 hours.
... The absorption rate is approximately 42%. From the blood level after intravenous administration a half-life of 200 min was calculated.
PMID:6933924 Klocking HP et al; Arch Toxicol Suppl 4: 312-4 (1980)
Cytisine is a low efficacy partial agonist of 4-2 nicotinic acetylcholine receptors. These which are believed to be central to the effect of nicotine (NIC) on the reward pathway and facilitate addiction. Cytisine reduces the effects of NIC on dopamine release in the mesolimbic system when given alone, while simultaneously attenuating NIC withdrawal symptoms that accompany cessation attempts.
Cytisine is an agonist of the cholinoreceptors in the vegetative ganglia and belongs to the group of the gangliostimulating drugs. It excites the nicotine-sensitive cholinoreceptors of the postsynaptic membranes in the vegetative ganglia, chromaffin cells in the molecular part of the suprarenal gland and sinocarotid reflexogenic zone, which results in excitation of the respiratory center, predominantly through the reflexes, simulation of adrenaline release by the medullar part of the suprarenal glands and a rise in the blood pressure. After its absorption in the gastrointestinal tract, cytisine plays the part of a nicotine-substitute substance which decreases the period of interaction between nicotine and the corresponding receptors. This in turn leads to a gradual decrease and interruption of the smokers' psychic and physical nicotine dependence.
Sopharma AD; Patient Information for Tabex tablets (2006).
A family of genes has been identified that encodes subunits of nicotinic acetylcholine receptors (nAChRs) and is expressed in the nervous system. Functional neuronal nAChRs can be expressed in Xenopus oocytes by injection of RNA encoding 1 of 2 different beta-subunits (beta 2, beta 4) in pairwise combination with RNA encoding 1 of 3 different alpha-subunits (alpha 2, alpha 3, alpha 4). We examined the sensitivity of these 6 different alpha- beta-subunit combinations to the nicotinic agonists ACh, nicotine, cytisine, and 1,1-dimethyl-4-phenylpiperazinium (DMPP). Each subunit combination displayed a distinct pattern of sensitivity to these 4 agonists. The alpha 2 beta 2 combination was 5-fold more sensitive to nicotine than to acetylcholine, while the alpha 3 beta 2 combination was 17-fold less sensitive to nicotine than to ACh, and the alpha 3 beta 4 combination was equally sensitive to both nicotine and ACh. nAChRs composed of alpha 2, alpha 3, or alpha 4 in combination with beta 2 were 14-100-fold less sensitive to cytisine than to ACh. In contrast, nAChRs composed of alpha 2, alpha 3, or alpha 4 in combination with beta 4 were 3-17-fold more sensitive to cytisine than to ACh. The alpha 2 beta 2, alpha 3 beta 2, and alpha 3 beta 4 combinations were each equally sensitive to DMPP and ACh, while the alpha 2 beta 4, alpha 4 beta 2, and alpha 4 beta 4 combinations were 4-24-fold less sensitive to DMPP than to ACh. We also demonstrated that these differences are neither a consequence of variation in the relative amounts of RNA injected nor an artifact of oocyte expression. The oocyte system can accurately express ligand-gated ion channels because mouse muscle nAChRs expressed in oocytes display pharmacological properties similar to those reported for these receptors expressed on BC3H-1 cells. We conclude that both the alpha- and the beta-subunits contribute to the pharmacological characteristics of neuronal nAChRs.
PMID:1705971 Luetje CW, Patrick J; J Neurosci. 11(3):837-45 (1991)
Neuronal nicotinic acetylcholine receptors subserve predominantly modulatory roles in the brain, making them attractive therapeutic targets. Natural products provide key leads in the quest for nicotinic receptor subtype-selective compounds. Cytisine, found in Leguminosae spp., binds with high affinity to alpha4beta2* nicotinic receptors. We have compared the effect of C3 and C5 halogenation of cytisine and methylcytisine (MCy) on their interaction with native rat nicotinic receptors. 3-Bromocytisine (3-BrCy) and 3-iodocytisine (3-ICy) exhibited increased binding affinity (especially at alpha7 nicotinic receptors; Ki approximately 0.1 microM) and functional potency, whereas C5-halogenation was detrimental. 3-BrCy and 3-ICy were more potent than cytisine at evoking (3)H dopamine release from striatal slices (EC50 approximately 11 nM), (3)H noradrenaline release from hippocampal slices (EC50 approximately 250 nM), increases in intracellular Ca2+ in PC12 cells and inward currents in Xenopus oocytes expressing human alpha3beta4 nicotinic receptor (EC50 approximately 2 microM). These compounds were also more efficacious than cytisine. C3-halogenation of cytisine is proposed to stabilize the open conformation of the nicotinic receptor but does not enhance subtype selectivity.
PMID:16563372 Abin-Carriquiry JA et al; Eur J Pharmacol. 536(1-2):1-11(2006)
Cytisine, a partial agonist that binds with high affinity to the alpha4beta2 nicotinic acetylcholine receptor...
PMID:21991893 West R et al; N Engl J Med 365: 1193-1200 (2011)
For more Mechanism of Action (Complete) data for CYTISINE (7 total), please visit the HSDB record page.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38934
Submission : 2023-10-11
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39649
Submission : 2024-03-12
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35908
Submission : 2021-05-06
Status : Active
Type : II




 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
About the Company : Aktin Pharm is a dedicated pharmaceutical company focused on delivering high-quality, reliable healthcare solutions across various therapeutic areas. Driven by a commitment to inno...

About the Company : Chengdu Chen Green Biotechnology (Chen Green) is a company specializing in plant extracts, pharmaceutical raw materials, food additives and dietary supplements manufacturers and wh...

About the Company : Hunan Bitian Technology Co.,Ltd is dedicate to R&D, Manufacturing and Distribution of Natural Ingredients, Natural Colors, Natrual Fruit & Vegetable Powder, Cosmetic Materials and ...

About the Company : Founded in 1980, Sichuan Xieli Pharmaceutical Co., Ltd. operates with the guiding principle of "collaborating to build a healthy enterprise." The company specializes in the cultiva...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Injectable / Parenteral
Grade : Parenteral
Application : Thickeners and Stabilizers
Excipient Details : Sucrose is used to stabilize proteins, lipids, carbohydrates, ADCs & vaccines. It is also used as a cryopreservative in cell-based bioprocesses.
Pharmacopoeia Ref : USP NF, EP, JP, ChP
Technical Specs : Low Endotoxin, Low Metals
Ingredient(s) : Sucrose
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Injectable / Parenteral
Grade : Biopharma Grade
Application : Thickeners and Stabilizers
Excipient Details : Used as carbon source in cell cultures in upstream, cryoprotectant and stabilizer in lyophilized formulations.
Pharmacopoeia Ref : On Request
Technical Specs : Low bacteria endotoxins, low bioburden (TAMC/TYMC). Customised packaging (from grs to kilograms)
Ingredient(s) : Saccharose Excipient
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Syrup
Grade : Oral
Brand Name : Sucrose Multi-Compendial
Application : Taste Masking
Excipient Details : A & C's Sucrose multi-compendial is a pharmaceutical excipient grade meeting the current specifications of USP-NF, EP and JP monographs.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Taste Masking
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : Pullulan is a pharmaceutical excipient that acts as an oxygen barrier polymer, a pharmaceutical binder, a filler and an ODF polymer.
Pharmacopoeia Ref : USP-NF, JP, EP
Technical Specs : Naturally occurring water-soluble neutral polysaccharide produced from starch syrup by fermentation.
Ingredient(s) : Pullulan
Dosage Form : Capsule
Grade : Oral
Application : Taste Masking
Excipient Details : Ready mix sugar coating system.
Pharmacopoeia Ref : USP, EP, JP & having US DMF
Technical Specs : Sprayable sugar coating system for solid oral dosage form
Ingredient(s) : Hydroxypropyl Methyl Cellulose
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Granule / Pellet
Grade : Oral
Application : Fillers, Diluents & Binders
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Cream / Lotion / Ointment
Grade : Topical
Application : Topical
Excipient Details : Menthol is used in nasal sprays, topical gel patches and creams, and ointments.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
32
PharmaCompass offers a list of Cytisine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Cytisine manufacturer or Cytisine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Cytisine manufacturer or Cytisine supplier.
PharmaCompass also assists you with knowing the Cytisine API Price utilized in the formulation of products. Cytisine API Price is not always fixed or binding as the Cytisine Price is obtained through a variety of data sources. The Cytisine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Cytisine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Cytisine, including repackagers and relabelers. The FDA regulates Cytisine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Cytisine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Cytisine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Cytisine supplier is an individual or a company that provides Cytisine active pharmaceutical ingredient (API) or Cytisine finished formulations upon request. The Cytisine suppliers may include Cytisine API manufacturers, exporters, distributors and traders.
click here to find a list of Cytisine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Cytisine DMF (Drug Master File) is a document detailing the whole manufacturing process of Cytisine active pharmaceutical ingredient (API) in detail. Different forms of Cytisine DMFs exist exist since differing nations have different regulations, such as Cytisine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Cytisine DMF submitted to regulatory agencies in the US is known as a USDMF. Cytisine USDMF includes data on Cytisine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Cytisine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Cytisine suppliers with USDMF on PharmaCompass.
Cytisine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Cytisine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Cytisine GMP manufacturer or Cytisine GMP API supplier for your needs.
A Cytisine CoA (Certificate of Analysis) is a formal document that attests to Cytisine's compliance with Cytisine specifications and serves as a tool for batch-level quality control.
Cytisine CoA mostly includes findings from lab analyses of a specific batch. For each Cytisine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Cytisine may be tested according to a variety of international standards, such as European Pharmacopoeia (Cytisine EP), Cytisine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Cytisine USP).
