
Synopsis
Synopsis
0
JDMF
0
EU WC
0
VMF
0
Australia

0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
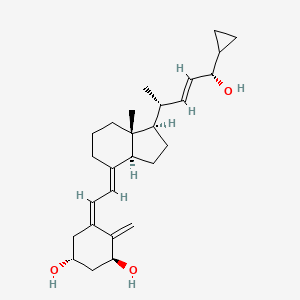

1. 1,24(oh)2-22-ene-24-cyclopropyl D3
2. Calcipotriene
3. Daivonex
4. Dovonex
5. Heximar Win Care
6. Mc 903
7. Mc-903
8. Pri-2205
9. Psorcutan
10. Sorilux
1. Calcipotriene
2. Dovonex
3. Daivonex
4. Sorilux
5. 112965-21-6
6. Calciptriol
7. Psorcutan
8. Calcipotriol Hydrate
9. Mc 903
10. Calcitrene
11. Mc-903
12. Calcipotriene [usan]
13. Calcipotriol [inn]
14. Calcipotriol Anhydrous
15. Chebi:50749
16. Dovonex (tn)
17. 112828-00-9
18. Pri-2201
19. Calcipotriol (jan)
20. 143nq3779b
21. (1r,3s,5z)-5-[(2e)-2-[(1r,3as,7ar)-1-[(e,2r,5s)-5-cyclopropyl-5-hydroxypent-3-en-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol
22. Ncgc00167465-01
23. Calcipotriol [jan]
24. Dsstox_cid_26648
25. Dsstox_rid_81793
26. Dsstox_gsid_46648
27. Divonex
28. (22e)-(24s)-1alpha,24-dihydroxy-26,27-cyclo-22,23-didehydrovitamin D3 / (22e)-(24s)-1alpha,24-dihydroxy-26,27-cyclo-22,23-didehydrocholecalciferol / Calcipotriol
29. (5z,7e,22e)-(1s,3r,24s)-26,27-cyclo-9,10-seco-5,7,10(19),22-cholestatetraene-1,3,24-triol
30. Smr000466353
31. Cas-112965-21-6
32. Sr-01000762910
33. Ccris 7700
34. Unii-143nq3779b
35. Pri 2201
36. Calcipotriene (usp)
37. Bms-181161
38. Calcipotrienemc 903
39. Calcipotriene [mi]
40. Epitope Id:114242
41. Schembl2853
42. Calcipotriene [vandf]
43. Calcipotriol [mart.]
44. Mls000759467
45. Mls001424130
46. Calcipotriol [who-dd]
47. Calcipotriene [usp-rs]
48. Gtpl2778
49. Chembl1200666
50. Dtxsid0046648
51. Amy2864
52. 1s19
53. Hms2051n11
54. Hms2089j08
55. Hms3269p03
56. Hms3413d04
57. Hms3677d04
58. Hms3713k08
59. Mc903
60. Calcipotriene [orange Book]
61. Calcipotriol [ep Monograph]
62. Ex-a4430
63. Zinc3921872
64. Tox21_112469
65. Bdbm50369964
66. Calcipotriene [usp Monograph]
67. Lmst03020106
68. S3739
69. Akos015855239
70. Tox21_112469_1
71. Ccg-100949
72. Cs-0387
73. Db02300
74. Nc00199
75. Stf-115469
76. Ncgc00167465-02
77. (5z,7e,22e,24s)-24-cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-1alpha,3beta,24-triol
78. As-56390
79. Cpd000466353
80. Hy-10001
81. D01125
82. U-0267
83. Ab00698343-05
84. 828c009
85. Q155683
86. Sr-01000762910-3
87. Sr-01000762910-4
88. (1?,3?,5z,7e,22e,24s)-24-cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-1,3,24-triol
89. (1s,3r,5z,7e,14beta,17alpha,22e,24s)-26,27-cyclo-9,10-secocholesta-5,7,10,22-tetraene-1,3,24-triol
90. (5z,7e,22e,24s)-24-cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-1.alpha.,3.beta.,24-triol
91. (5z,7e,24s)-26,27-cyclo-9,10-secocholesta-5,7,10(19),22-tetraene-1alpha,3beta,24-triol
92. 9,10-secochola-5,7,10(19),22-tetraene-1,3,24-triol, 24-cyclopropyl-, (1.alpha.,3.beta.,5z,7e,22e,24s)-
93. 9,10-secochola-5,7,10(19),22-tetraene-1,3,24-triol, 24-cyclopropyl-, (1alpha,3beta,5z,7e,22e,24s)-
| Molecular Weight | 412.6 g/mol |
|---|---|
| Molecular Formula | C27H40O3 |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 412.29774513 g/mol |
| Monoisotopic Mass | 412.29774513 g/mol |
| Topological Polar Surface Area | 60.7 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 743 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Calcipotriene |
| PubMed Health | Calcipotriene (On the skin) |
| Drug Classes | Antipsoriatic |
| Drug Label | Dovonex (calcipotriene solution) Scalp Solution, 0.005% is a colorless topical solution containing calcipotriene monohydrate, a synthetic vitamin D3 derivative, for topical dermatologic use.Chemically, calcipotriene monohydrate is (5Z,7E,22E,24S)... |
| Active Ingredient | Calcipotriene |
| Dosage Form | Ointment; Cream; Solution |
| Route | Topical |
| Strength | 0.005% |
| Market Status | Prescription |
| Company | Glenmark Generics; Fougera Pharms; Tolmar; G And W Labs |
| 2 of 6 | |
|---|---|
| Drug Name | Dovonex |
| PubMed Health | Calcipotriene (On the skin) |
| Drug Classes | Antipsoriatic |
| Drug Label | Dovonex (calcipotriene solution) Scalp Solution, 0.005% is a colorless topical solution containing calcipotriene monohydrate, a synthetic vitamin D3 derivative, for topical dermatologic use.Chemically, calcipotriene monohydrate is (5Z,7E,22E,24S)... |
| Active Ingredient | Calcipotriene |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.005% |
| Market Status | Prescription |
| Company | Leo Pharma As |
| 3 of 6 | |
|---|---|
| Drug Name | Sorilux |
| PubMed Health | Calcipotriene (On the skin) |
| Drug Classes | Antipsoriatic |
| Drug Label | SORILUX Foam contains the compound calcipotriene, a synthetic vitamin D3 analog.Chemically, calcipotriene is (5Z,7E,22E,24S)-24-cyclopropyl-9,10-secochola-5,7,10(19), 22-tetraene-1,3,24-triol. The structural formula is represented below:Molecular... |
| Active Ingredient | Calcipotriene |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 0.005% |
| Market Status | Prescription |
| Company | Stiefel Labs |
| 4 of 6 | |
|---|---|
| Drug Name | Calcipotriene |
| PubMed Health | Calcipotriene (On the skin) |
| Drug Classes | Antipsoriatic |
| Drug Label | Dovonex (calcipotriene solution) Scalp Solution, 0.005% is a colorless topical solution containing calcipotriene monohydrate, a synthetic vitamin D3 derivative, for topical dermatologic use.Chemically, calcipotriene monohydrate is (5Z,7E,22E,24S)... |
| Active Ingredient | Calcipotriene |
| Dosage Form | Ointment; Cream; Solution |
| Route | Topical |
| Strength | 0.005% |
| Market Status | Prescription |
| Company | Glenmark Generics; Fougera Pharms; Tolmar; G And W Labs |
| 5 of 6 | |
|---|---|
| Drug Name | Dovonex |
| PubMed Health | Calcipotriene (On the skin) |
| Drug Classes | Antipsoriatic |
| Drug Label | Dovonex (calcipotriene solution) Scalp Solution, 0.005% is a colorless topical solution containing calcipotriene monohydrate, a synthetic vitamin D3 derivative, for topical dermatologic use.Chemically, calcipotriene monohydrate is (5Z,7E,22E,24S)... |
| Active Ingredient | Calcipotriene |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.005% |
| Market Status | Prescription |
| Company | Leo Pharma As |
| 6 of 6 | |
|---|---|
| Drug Name | Sorilux |
| PubMed Health | Calcipotriene (On the skin) |
| Drug Classes | Antipsoriatic |
| Drug Label | SORILUX Foam contains the compound calcipotriene, a synthetic vitamin D3 analog.Chemically, calcipotriene is (5Z,7E,22E,24S)-24-cyclopropyl-9,10-secochola-5,7,10(19), 22-tetraene-1,3,24-triol. The structural formula is represented below:Molecular... |
| Active Ingredient | Calcipotriene |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 0.005% |
| Market Status | Prescription |
| Company | Stiefel Labs |
For the treatment of moderate plaque psoriasis in adults.
Treatment of psoriasis
Calcipotriene is a synthetic analog of vitamin D. In humans, the natural supply of vitamin D depends mainly on exposure to the ultraviolet rays of the sun for conversion of 7-dehydrocholesterol to vitamin D3 (cholecalciferol) in the skin.
Dermatologic Agents
Drugs used to treat or prevent skin disorders or for the routine care of skin. (See all compounds classified as Dermatologic Agents.)
D05AX52
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D05 - Antipsoriatics
D05A - Antipsoriatics for topical use
D05AX - Other antipsoriatics for topical use
D05AX02 - Calcipotriol
Absorption
Clinical studies with radiolabeled ointment indicate that approximately 6% (+3%, SD) of the applied dose of calcipotriene is absorbed systemically when the ointment is applied topically to psoriasis plaques or 5% (+2.6%, SO) when applied to normal skin.
Route of Elimination
The active form of the vitamin, 1,25-dihydroxy vitamin D3 (calcitriol), is known to be recycled via the liver and excreted in the bile. There is evidence that maternal 1,25-dihydroxy vitamin D3 (calcitriol) may enter the fetal circulation, but it is not known whether it is excreted in human milk.
Hepatic. Calcipotriene metabolism following systemic uptake is rapid, and occurs via a similar pathway to the natural hormone. The primary metabolites are much less potent than the parent compound.
The precise mechanism of calcipotriol in remitting psoriasis is not well-understood, however, it has been shown to have comparable affinity with calcitriol for the Vitamin D receptor while being less than 1% the activity in regulating calcium metabolism. The Vitamin D receptor (VDR) belongs to the steroid/thyroid receptor superfamily, and is found on the cells of many different tissues including the thyroid, bone, kindney, and T cells of the immune system. T cells are known to play a role in psoriasis and are believed to undergo modulation of gene expression with binding of calcipotriol to the VDR. This modulation is thought to affect gene products related to cell differentiation and proliferation.
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Century has been an API manufacturer for over 40 years & is the partner of choice for multipurpose custom manufacturing projects.
Century has been an API manufacturer for over 40 years & is the partner of choice for multipurpose custom manufacturing projects.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

GDUFA
DMF Review : Reviewed
Rev. Date : 2012-11-30
Pay. Date : 2012-11-13
DMF Number : 16958
Submission : 2003-11-14
Status : Active
Type : II

Certificate Number : R1-CEP 2014-016 - Rev 01
Issue Date : 2021-12-20
Type : Chemical
Substance Number : 2284
Status : Valid

| Available Reg Filing : CA, ASMF |
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

Certificate Number : R1-CEP 2007-223 - Rev 03
Issue Date : 2022-01-04
Type : Chemical
Substance Number : 2011
Status : Valid

| Available Reg Filing : CA, ASMF |
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8308
Submission : 1989-11-01
Status : Inactive
Type : II

Certificate Number : R1-CEP 2005-148 - Rev 01
Issue Date : 2013-03-13
Type : Chemical
Substance Number : 2011
Status : Valid

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8312
Submission : 1989-11-27
Status : Inactive
Type : II

Certificate Number : R1-CEP 2005-145 - Rev 01
Issue Date : 2013-04-09
Type : Chemical
Substance Number : 2284
Status : Valid

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8714
Submission : 1990-08-24
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8715
Submission : 1990-08-24
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
74
PharmaCompass offers a list of Calcipotriol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Calcipotriol manufacturer or Calcipotriol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Calcipotriol manufacturer or Calcipotriol supplier.
PharmaCompass also assists you with knowing the Calcipotriol API Price utilized in the formulation of products. Calcipotriol API Price is not always fixed or binding as the Calcipotriol Price is obtained through a variety of data sources. The Calcipotriol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Daivonex manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Daivonex, including repackagers and relabelers. The FDA regulates Daivonex manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Daivonex API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Daivonex manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Daivonex supplier is an individual or a company that provides Daivonex active pharmaceutical ingredient (API) or Daivonex finished formulations upon request. The Daivonex suppliers may include Daivonex API manufacturers, exporters, distributors and traders.
click here to find a list of Daivonex suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Daivonex DMF (Drug Master File) is a document detailing the whole manufacturing process of Daivonex active pharmaceutical ingredient (API) in detail. Different forms of Daivonex DMFs exist exist since differing nations have different regulations, such as Daivonex USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Daivonex DMF submitted to regulatory agencies in the US is known as a USDMF. Daivonex USDMF includes data on Daivonex's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Daivonex USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Daivonex suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Daivonex Drug Master File in Korea (Daivonex KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Daivonex. The MFDS reviews the Daivonex KDMF as part of the drug registration process and uses the information provided in the Daivonex KDMF to evaluate the safety and efficacy of the drug.
After submitting a Daivonex KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Daivonex API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Daivonex suppliers with KDMF on PharmaCompass.
A Daivonex CEP of the European Pharmacopoeia monograph is often referred to as a Daivonex Certificate of Suitability (COS). The purpose of a Daivonex CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Daivonex EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Daivonex to their clients by showing that a Daivonex CEP has been issued for it. The manufacturer submits a Daivonex CEP (COS) as part of the market authorization procedure, and it takes on the role of a Daivonex CEP holder for the record. Additionally, the data presented in the Daivonex CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Daivonex DMF.
A Daivonex CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Daivonex CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Daivonex suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Daivonex as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Daivonex API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Daivonex as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Daivonex and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Daivonex NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Daivonex suppliers with NDC on PharmaCompass.
Daivonex Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Daivonex GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Daivonex GMP manufacturer or Daivonex GMP API supplier for your needs.
A Daivonex CoA (Certificate of Analysis) is a formal document that attests to Daivonex's compliance with Daivonex specifications and serves as a tool for batch-level quality control.
Daivonex CoA mostly includes findings from lab analyses of a specific batch. For each Daivonex CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Daivonex may be tested according to a variety of international standards, such as European Pharmacopoeia (Daivonex EP), Daivonex JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Daivonex USP).
