
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
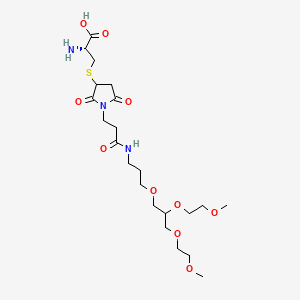

1. Unii-by4tsk952y
2. Damoctocog Alfa Pegol [inn]
3. By4tsk952y
4. 1363853-26-2
5. Des-(743-1636)-(1804-(s-(1-(3-((3-(2,3-bis(omega-methoxypoly(oxyethylene))propoxy)propyl)amino)-3-oxopropyl)-2,5-dioxopyrrolidin-3-yl)-l-cysteine)(k>c))human Coagulation Factor Viii
6. Q27274954
| Molecular Weight | 537.6 g/mol |
|---|---|
| Molecular Formula | C22H39N3O10S |
| XLogP3 | -4.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 22 |
| Exact Mass | 537.23561562 g/mol |
| Monoisotopic Mass | 537.23561562 g/mol |
| Topological Polar Surface Area | 201 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 685 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for use in previously treated adults and adolescents (12 years of age and above) with hemophilia A (congenital Factor VIII deficiency) for: On-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes.
FDA Label
Treatment and prophylaxis of bleeding in previously treated patients 12 years of age with haemophilia A (congenital factor VIII deficiency).
Treatment of hereditary factor VIII deficiency
This agent is engineered to prolong blood FVIII activity while maintaining coagulation activity using PEGylation, where a PEG (Polyethylene glycol) molecule is continually attached to the factor VIII protein at a specific site,. This prevents and controls bleeding episodes associated with hemophilia A. The aPTT is prolonged in people diagnosed with hemophilia A. The determination of aPTT is a conventional in vitro assay for assessing the biological activity of Factor VIII. Treatment with damoctogog alfa pegol normalizes the aPTT similar to that achieved with plasma-derived Factor VIII. The administration of this agent increases plasma levels of Factor VIII and can temporarily correct the coagulation defect that exists in hemophilia A patients.
B02BD02
Absorption
After a single dose, AUC (area under the curve) was 1640 550 with a dose of 25 IU/kg.
Clearance
142 33 mL/h on with a dose of 25 IU/kg and 121 53 mL/h with a dose of 60 IU/kg.
18.6 4.6h after a single dose
This drug is a site-specifically PEGylated recombinant antihemophilic factor, which temporarily replaces the missing coagulation Factor VIII. The site-specific PEGylation in the A3 domain reduces binding to the physiological Factor VIII clearance receptors resulting in a longer half-life and increased AUC (area under the curve). The active protein, prior to conjugation is a recombinant B-domain deleted human coagulation Factor VIII (BDD-rFVIII) produced by recombinant DNA technology in Baby Hamster Kidney (BHK) cells. Damoctagol alfa pegol is manufactured by site-specific conjugation of the BDD-rFVIII variant K1804C at the cysteine amino acid position 1804 (within the A3 domain) with a single maleimide-derivatized, 60 kilodalton (kDa) branched PEG (two 30 kDa PEG) moiety. The A3 domain was identified and selected for conjugation to provide both a continual coagulation activity and high PEGylation efficiency.
Global Sales Information

ABOUT THIS PAGE
16
PharmaCompass offers a list of Damoctocog Alfa Pegol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Damoctocog Alfa Pegol manufacturer or Damoctocog Alfa Pegol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Damoctocog Alfa Pegol manufacturer or Damoctocog Alfa Pegol supplier.
PharmaCompass also assists you with knowing the Damoctocog Alfa Pegol API Price utilized in the formulation of products. Damoctocog Alfa Pegol API Price is not always fixed or binding as the Damoctocog Alfa Pegol Price is obtained through a variety of data sources. The Damoctocog Alfa Pegol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Damoctocog Alfa Pegol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Damoctocog Alfa Pegol, including repackagers and relabelers. The FDA regulates Damoctocog Alfa Pegol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Damoctocog Alfa Pegol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Damoctocog Alfa Pegol supplier is an individual or a company that provides Damoctocog Alfa Pegol active pharmaceutical ingredient (API) or Damoctocog Alfa Pegol finished formulations upon request. The Damoctocog Alfa Pegol suppliers may include Damoctocog Alfa Pegol API manufacturers, exporters, distributors and traders.
Damoctocog Alfa Pegol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Damoctocog Alfa Pegol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Damoctocog Alfa Pegol GMP manufacturer or Damoctocog Alfa Pegol GMP API supplier for your needs.
A Damoctocog Alfa Pegol CoA (Certificate of Analysis) is a formal document that attests to Damoctocog Alfa Pegol's compliance with Damoctocog Alfa Pegol specifications and serves as a tool for batch-level quality control.
Damoctocog Alfa Pegol CoA mostly includes findings from lab analyses of a specific batch. For each Damoctocog Alfa Pegol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Damoctocog Alfa Pegol may be tested according to a variety of international standards, such as European Pharmacopoeia (Damoctocog Alfa Pegol EP), Damoctocog Alfa Pegol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Damoctocog Alfa Pegol USP).
