


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
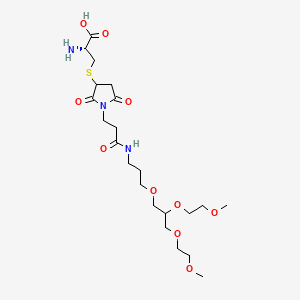

1. Unii-by4tsk952y
2. Damoctocog Alfa Pegol [inn]
3. By4tsk952y
4. 1363853-26-2
5. Des-(743-1636)-(1804-(s-(1-(3-((3-(2,3-bis(omega-methoxypoly(oxyethylene))propoxy)propyl)amino)-3-oxopropyl)-2,5-dioxopyrrolidin-3-yl)-l-cysteine)(k>c))human Coagulation Factor Viii
6. Q27274954
| Molecular Weight | 537.6 g/mol |
|---|---|
| Molecular Formula | C22H39N3O10S |
| XLogP3 | -4.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 22 |
| Exact Mass | 537.23561562 g/mol |
| Monoisotopic Mass | 537.23561562 g/mol |
| Topological Polar Surface Area | 201 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 685 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for use in previously treated adults and adolescents (12 years of age and above) with hemophilia A (congenital Factor VIII deficiency) for: On-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes.
FDA Label
Treatment and prophylaxis of bleeding in previously treated patients 12 years of age with haemophilia A (congenital factor VIII deficiency).
Treatment of hereditary factor VIII deficiency
This agent is engineered to prolong blood FVIII activity while maintaining coagulation activity using PEGylation, where a PEG (Polyethylene glycol) molecule is continually attached to the factor VIII protein at a specific site,. This prevents and controls bleeding episodes associated with hemophilia A. The aPTT is prolonged in people diagnosed with hemophilia A. The determination of aPTT is a conventional in vitro assay for assessing the biological activity of Factor VIII. Treatment with damoctogog alfa pegol normalizes the aPTT similar to that achieved with plasma-derived Factor VIII. The administration of this agent increases plasma levels of Factor VIII and can temporarily correct the coagulation defect that exists in hemophilia A patients.
B02BD02
Absorption
After a single dose, AUC (area under the curve) was 1640 550 with a dose of 25 IU/kg.
Clearance
142 33 mL/h on with a dose of 25 IU/kg and 121 53 mL/h with a dose of 60 IU/kg.
18.6 4.6h after a single dose
This drug is a site-specifically PEGylated recombinant antihemophilic factor, which temporarily replaces the missing coagulation Factor VIII. The site-specific PEGylation in the A3 domain reduces binding to the physiological Factor VIII clearance receptors resulting in a longer half-life and increased AUC (area under the curve). The active protein, prior to conjugation is a recombinant B-domain deleted human coagulation Factor VIII (BDD-rFVIII) produced by recombinant DNA technology in Baby Hamster Kidney (BHK) cells. Damoctagol alfa pegol is manufactured by site-specific conjugation of the BDD-rFVIII variant K1804C at the cysteine amino acid position 1804 (within the A3 domain) with a single maleimide-derivatized, 60 kilodalton (kDa) branched PEG (two 30 kDa PEG) moiety. The A3 domain was identified and selected for conjugation to provide both a continual coagulation activity and high PEGylation efficiency.


