

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
API
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Data Compilation #PharmaFlow
0
Weekly News Recap #Phispers

1. Nubeqa
2. Odm-201
3. Orm-16497
4. Orm-16555
1. Odm-201
2. 1297538-32-9
3. Bay-1841788
4. Nubeqa
5. Bay1841788
6. Darolutamide [usan]
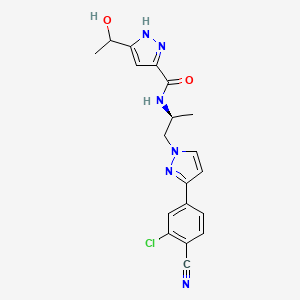
7. N-((s)-1-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl)propan-2-yl)-5-(1-hydroxyethyl)-1h-pyrazole-3-carboxamide
8. Bay 1841788
9. X05u0n2rco
10. Odm-201;bay-1841788
11. 1h-pyrazole-3-carboxamide, N-((1s)-2-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl)-1-methylethyl)-5-(1-hydroxyethyl)-
12. N-((s)-1-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl)-propan-2-yl)-3-(1-hydroxyethyl)-1h-pyrazole-5-carboxamide
13. Odm201
14. 1h-pyrazole-3-carboxamide, N-[(1s)-2-[3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl]-1-methylethyl]-5-(1-hydroxyethyl)-
15. Nebeqa (tn)
16. Darolutamide [mi]
17. Odm-201(darolutamide)
18. Darolutamide [inn]
19. Darolutamide [jan]
20. Unii-x05u0n2rco
21. Darolutamide (odm-201)
22. Darolutamide [who-dd]
23. Darolutamide (jan/usan/inn)
24. Schembl1814935
25. Chembl4297185
26. Schembl13733117
27. Gtpl10439
28. Ex-a759
29. Bdbm309979
30. Darolutamide [orange Book]
31. Dtxsid101027953
32. Example 56 [us9657003]
33. N-[(2s)-1-[3-(3-chloro-4-cyanophenyl)pyrazol-1-yl]propan-2-yl]-5-(1-hydroxyethyl)-1h-pyrazole-3-carboxamide
34. Bdbm50556205
35. Mfcd29472270
36. Nsc825331
37. Akos030526387
38. Ccg-268640
39. Cs-5174
40. Db12941
41. Nsc-825331
42. Ncgc00484078-01
43. Ac-32628
44. As-75032
45. Bay-1841788)
46. Hy-16985
47. S7559
48. J3.501.129c
49. D11045
50. Us9657003, 56
51. A888821
52. J-690121
53. Q25091391
54. 1-[(2s)-2-butanyl]-n-[(4,6-dimethyl-2-oxo-1,2-dihydro-3-pyridinyl)methyl]-3-methyl-6-[6-(1-piperazinyl)-3-pyridinyl]-1h-indole-4-car Boxamide
55. N-((2s)-1-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol- 1-yl)propan-2-yl)-5-((1rs)-1-hydroxyethyl)-1h-pyrazole- 3-carboxamide
56. N-((2s)-1-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl)propan-2-yl)-5-((1rs)-1-hydroxyethyl)-1h-pyrazole-3-carboxamide
57. N-((s)-1-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol -1-yl)propan-2-yl)-3-(1-hydroxyethyl)-1h-pyrazole-5-carboxamide
58. N-((s)-1-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl)propan-2-yl)-5-(1-hydroxy-ethyl)-1h-pyrazole-3-carboxamide
59. N-[(2s)-1-[3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl]propan-2-yl]-5-(1-hydroxyethyl)-1h-pyrazole-3-carboxamide
| Molecular Weight | 398.8 g/mol |
|---|---|
| Molecular Formula | C19H19ClN6O2 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 398.1258016 g/mol |
| Monoisotopic Mass | 398.1258016 g/mol |
| Topological Polar Surface Area | 120 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 598 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
This drug is indicated for the treatment of patients diagnosed with non-metastatic and castrate-resistant prostate cancer.
FDA Label
Nubeqa is indicated for the treatment of adult men with non metastatic castration resistant prostate cancer (nmCRPC) who are at risk of developing metastatic disease.
Darolutamide, through its downstream effects on cancer cell growth, treats castrate-resistant prostate cancer. It inhibits cancer cell growth and markedly lowers prostate specific antigen (PSA) levels through potent androgen receptor antagonism.
L02BB
L - Antineoplastic and immunomodulating agents
L02 - Endocrine therapy
L02B - Hormone antagonists and related agents
L02BB - Anti-androgens
L02BB06 - Darolutamide
Absorption
Darolutamide is absorbed in the gastrointestinal tract. In the fasted state, peak concentrations are reached within 3-5 hours, and within 3-8 hours in the fed state. Median Tmax is between 3-6 hours.The average darolutamide steady-state peak plasma concentration after a 600 mg twice daily dose is approximately 4.79 mg/L. The Cmax is attained approximately 4 hours after administration of a single 600 mg oral dose. The AUC 0-12h is approximately 52.82 hg/mL. **Effects of food** The absolute bioavailability of darolutamide is approximately 30% after fasting and taking a single 300 mg dose. Steady-state concentrations are attained between 2 and 5 days after repeated administration with food. The bioavailability of darolutamide increases by 2.0 to 2.5 times when it is given with food.
Route of Elimination
In a pharmacokinetic study, a radiolabeled dose of darolutamide in an oral solution showed that 63.4% of darolutamide-related material was excreted in the urine (7% of which was unchanged drug) and 32.4% in the feces (with 30% unchanged drug).
Volume of Distribution
After intravenous administration, the apparent volume of distribution of darolutamide is about 119L.
Clearance
The clearance of darolutamide after an intravenous dose is 116 mL/min (39.7%).
Darolutamide is mainly metabolized by the CYP3A4 hepatic microsomal enzyme in addition to UGT1A9 and UGT1A1. The main active metabolite keto-darolutamide in found in the plasma at 2 times the concentration of darolutamide.
The half-life of darolutamide and its active metabolite, keto-darolutamide is about 20 hours. A phase 1 study determined a terminal half life ranging between 10-15 hours.
The actions of androgens on androgen receptors (AR) potentiate the growth and survival of prostate cancer cells. Darolutamide competitively inhibits androgens from binding to their receptors, inhibiting AR nuclear translocation, as well as AR-mediated transcription. The end result of these processes is a decrease in prostate cancer cell proliferation and tumor size. Its main metabolite, keto-darolutamide, shows similar pharmacological activity to the parent drug, darolutamide. Darolutamide has been found to bind more tightly to the AR receptor than [apalutamide] and [enzalutamide], which are other androgen receptor antagonists. Darolutamide can act as a progesterone receptor (PR) antagonist in the laboratory setting with approximately 1% activity when compared to its actions at the androgen receptor. The clinical relevance is not known at this time.
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
 Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..

GDUFA
DMF Review : Reviewed
Rev. Date : 2023-04-20
Pay. Date : 2023-03-30
DMF Number : 37912
Submission : 2023-03-30
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2023-06-13
Pay. Date : 2023-04-25
DMF Number : 38274
Submission : 2023-05-02
Status : Active
Type : II

NDC Package Code : 68554-0140
Start Marketing Date : 2019-07-30
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36697
Submission : 2022-02-28
Status : Active
Type : II

Date of Issue : 2022-09-02
Valid Till : 2025-05-05
Written Confirmation Number : WC-0349
Address of the Firm :

NDC Package Code : 54893-0107
Start Marketing Date : 2021-01-22
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2023-01-30
Pay. Date : 2023-01-09
DMF Number : 37585
Submission : 2022-11-30
Status : Active
Type : II

NDC Package Code : 54893-0107
Start Marketing Date : 2021-01-22
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : Complete
Rev. Date : 2023-04-20
Pay. Date : 2023-03-30
DMF Number : 37912
Submission : 2023-03-30
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2023-06-13
Pay. Date : 2023-04-25
DMF Number : 38274
Submission : 2023-05-02
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36697
Submission : 2022-02-28
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2023-01-30
Pay. Date : 2023-01-09
DMF Number : 37585
Submission : 2022-11-30
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Date of Issue : 2022-09-02
Valid Till : 2025-05-05
Written Confirmation Number : WC-0349
Address of the Firm : MIs. MSN Laboratories Private Limited, Unit-II, sv. No, 50, Kardanur (Village), ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registrant Name : Bayer Korea Ltd.
Registration Date : 2020-05-27
Registration Number : Su212-7-ND
Manufacturer Name : Fermion Oy
Manufacturer Address : Orioninkatu 2, Hanko FI-10900 Finland
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Nubeqa (darolutamide) is an oral androgen receptor inhibitor (ARi) with a distinct chemical structure that binds to the receptor with high affinity and exhibits strong antagonistic activity, thereby inhibiting the receptor function and the growth of prostate cancer cells.
Lead Product(s): Darolutamide,Docetaxel
Therapeutic Area: Oncology Brand Name: Nubeqa
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Bayer AG
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 23, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Darolutamide,Docetaxel
Therapeutic Area : Oncology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Bayer AG
Deal Size : Not Applicable
Deal Type : Not Applicable
Orion and Bayer Expand Clinical Development Program for Darolutamide in Prostate Cancer
Details : Nubeqa (darolutamide) is an oral androgen receptor inhibitor (ARi) with a distinct chemical structure that binds to the receptor with high affinity and exhibits strong antagonistic activity, thereby inhibiting the receptor function and the growth of pros...
Brand Name : Nubeqa
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 23, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Nubeqa (darolutamide) is an oral androgen receptor inhibitor (ARi) with a distinct chemical structure that binds to the receptor with high affinity and exhibits strong antagonistic activity, thereby inhibiting the receptor function and the growth of prostate cancer cells.
Lead Product(s): Darolutamide,Docetaxel
Therapeutic Area: Oncology Brand Name: Nubeqa
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 20, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Darolutamide,Docetaxel
Therapeutic Area : Oncology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Darolutamide Approved for Additional Prostate Cancer Indication in China
Details : Nubeqa (darolutamide) is an oral androgen receptor inhibitor (ARi) with a distinct chemical structure that binds to the receptor with high affinity and exhibits strong antagonistic activity, thereby inhibiting the receptor function and the growth of pros...
Brand Name : Nubeqa
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 20, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Nubeqa (darolutamide) is an oral androgen receptor inhibitor (ARi) with a distinct chemical structure that binds to the receptor with high affinity and exhibits strong antagonistic activity, thereby inhibiting the receptor function and the growth of prostate cancer cells.
Lead Product(s): Darolutamide,Docetaxel
Therapeutic Area: Oncology Brand Name: Nubeqa
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 01, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Darolutamide,Docetaxel
Therapeutic Area : Oncology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Darolutamide Receives EU Approval for Additional Indication in Prostate Cancer
Details : Nubeqa (darolutamide) is an oral androgen receptor inhibitor (ARi) with a distinct chemical structure that binds to the receptor with high affinity and exhibits strong antagonistic activity, thereby inhibiting the receptor function and the growth of pros...
Brand Name : Nubeqa
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 01, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Nubeqa (darolutamide) is an oral androgen receptor inhibitor (ARi) with a distinct chemical structure that binds to the receptor with high affinity and exhibits strong antagonistic activity, thereby inhibiting the receptor function and the growth of prostate cancer cells.
Lead Product(s): Darolutamide,Docetaxel
Therapeutic Area: Oncology Brand Name: Nubeqa
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Bayer AG
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable February 27, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Darolutamide,Docetaxel
Therapeutic Area : Oncology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Bayer AG
Deal Size : Not Applicable
Deal Type : Not Applicable
Darolutamide Receives Approval for Additional Prostate Cancer Indication in Japan
Details : Nubeqa (darolutamide) is an oral androgen receptor inhibitor (ARi) with a distinct chemical structure that binds to the receptor with high affinity and exhibits strong antagonistic activity, thereby inhibiting the receptor function and the growth of pros...
Brand Name : Nubeqa
Molecule Type : Small molecule
Upfront Cash : Not Applicable
February 27, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Nubeqa (darolutamide) is an oral androgen receptor inhibitor (ARi) with a distinct chemical structure that binds to the receptor with high affinity and exhibits strong antagonistic activity, thereby inhibiting the receptor function and the growth of prostate cancer cells.
Lead Product(s): Darolutamide,Docetaxel
Therapeutic Area: Oncology Brand Name: Nubeqa
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable January 27, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Darolutamide,Docetaxel
Therapeutic Area : Oncology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Nubeqa (darolutamide) is an oral androgen receptor inhibitor (ARi) with a distinct chemical structure that binds to the receptor with high affinity and exhibits strong antagonistic activity, thereby inhibiting the receptor function and the growth of pros...
Brand Name : Nubeqa
Molecule Type : Small molecule
Upfront Cash : Not Applicable
January 27, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Nubeqa (darolutamide) is an oral androgen receptor inhibitor with a unique chemical structure developed to inhibit the growth of cancer cells. FDA has approved its use in combination with docetaxel for treating metastatic hormone-sensitive prostate cancer.
Lead Product(s): Darolutamide,Docetaxel
Therapeutic Area: Oncology Brand Name: Nubeqa
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 08, 2022
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Darolutamide,Docetaxel
Therapeutic Area : Oncology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Nubeqa (darolutamide) is an oral androgen receptor inhibitor with a unique chemical structure developed to inhibit the growth of cancer cells. FDA has approved its use in combination with docetaxel for treating metastatic hormone-sensitive prostate cance...
Brand Name : Nubeqa
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 08, 2022
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The sNDA is based on positive results from pivotal Phase III ARASENS trial demonstrating a statistically significant improvement in overall survival for Nubeqa (darolutamide) plus androgen deprivation therapy and docetaxel in men with mHSPC compared to ADT plus docetaxel.
Lead Product(s): Darolutamide,Docetaxel
Therapeutic Area: Oncology Brand Name: Nubeqa
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Bayer AG
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 03, 2022
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Darolutamide,Docetaxel
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Bayer AG
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : The sNDA is based on positive results from pivotal Phase III ARASENS trial demonstrating a statistically significant improvement in overall survival for Nubeqa (darolutamide) plus androgen deprivation therapy and docetaxel in men with mHSPC compared to A...
Brand Name : Nubeqa
Molecule Type : Small molecule
Upfront Cash : Not Applicable
May 03, 2022
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Nubeqa (darolutamide) is an oral androgen receptor inhibitor with a unique chemical structure developed to inhibit the growth of cancer cells. FDA has approved its use in combination with docetaxel for treating metastatic hormone-sensitive prostate cancer.
Lead Product(s): Darolutamide,Docetaxel
Therapeutic Area: Oncology Brand Name: Nubeqa
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 24, 2022
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Darolutamide,Docetaxel
Therapeutic Area : Oncology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Orion Plans to Focus Research and Product Development - In The Future on Cancer and Pain
Details : Nubeqa (darolutamide) is an oral androgen receptor inhibitor with a unique chemical structure developed to inhibit the growth of cancer cells. FDA has approved its use in combination with docetaxel for treating metastatic hormone-sensitive prostate cance...
Brand Name : Nubeqa
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 24, 2022
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Nubeqa (darolutamide) is an oral androgen receptor inhibitor with a unique chemical structure developed to inhibit the growth of cancer cells. FDA has approved its use in combination with docetaxel for treating metastatic hormone-sensitive prostate cancer.
Lead Product(s): Darolutamide
Therapeutic Area: Oncology Brand Name: Nubeqa
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Bayer AG
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable February 08, 2022
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Darolutamide
Therapeutic Area : Oncology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Bayer AG
Deal Size : Not Applicable
Deal Type : Not Applicable
Orion and Bayer Are Expanding the Darolutamide Development Program in The Area of prostate C...
Details : Nubeqa (darolutamide) is an oral androgen receptor inhibitor with a unique chemical structure developed to inhibit the growth of cancer cells. FDA has approved its use in combination with docetaxel for treating metastatic hormone-sensitive prostate cance...
Brand Name : Nubeqa
Molecule Type : Small molecule
Upfront Cash : Not Applicable
February 08, 2022
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The ARASENS trial, Nubeqa (darolutamide) in combination with docetaxel and androgen deprivation therapy significantly increased overall survival for patients with metastatic hormone-sensitive prostate cancer.
Lead Product(s): Darolutamide,Docetaxel
Therapeutic Area: Oncology Brand Name: Nubeqa
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Bayer AG
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 03, 2021
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Darolutamide,Docetaxel
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Bayer AG
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : The ARASENS trial, Nubeqa (darolutamide) in combination with docetaxel and androgen deprivation therapy significantly increased overall survival for patients with metastatic hormone-sensitive prostate cancer.
Brand Name : Nubeqa
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 03, 2021
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : NUBEQA
Dosage Form : TABLET;ORAL
Dosage Strength : 300MG
Approval Date : 2019-07-30
Application Number : 212099
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Nubeqa
Dosage Form : Film-Coated Tablets
Dosage Strength : 300mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Nubeqa
Dosage Form :
Dosage Strength :
Packaging : 112
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Nubeqa
Dosage Form :
Dosage Strength :
Packaging : 112
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
We have 1 companies offering Darolutamide
Get in contact with the supplier of your choice:


LOOKING FOR A SUPPLIER?
